4876
A new class of easily synthesized manganese contrast agents for liver MRI
Sean William McRae1, Michael Cleary2, Francisco Martinez3, Ying Xia3, Peter Caravan2, Eric Gale2, John Ronald3, and Timothy Scholl3
1Medical Biophysics, Western University, London, ON, Canada, 2Massachusetts General Hospital, Boston, MA, United States, 3Western University, London, ON, Canada
1Medical Biophysics, Western University, London, ON, Canada, 2Massachusetts General Hospital, Boston, MA, United States, 3Western University, London, ON, Canada
Synopsis
Keywords: Contrast Agent, Molecular Imaging
This work investigated the performance of a novel class of Mn(II) T1 contrast agents made from a commercially available chelator with various lipophilic targeting moieties added to promote uptake through the human derived OATP1B1 and OATP1B3 transporters. Agent performance was assessed in vitro using cells engineered to express the human OATPs, and in vivo by assessing liver uptake in mice where other OATP isoforms are present. In vitro measurements suggested targeting of agents to human OATPs where in vivo imaging revealed strong contrast enhancement in murine livers, with no evident toxicity at a 0.1 mmol/kg dose.Introduction
In vivo molecular imaging requires highly sensitive imaging technologies paired with optimized molecularly targeted imaging probes. In the clinic, magnetic resonance imaging (MRI) is often the standard for detection of macroscopic pathologies but due to its limited inherent sensitivity it often requires administration of gadolinium probes to compensate. One such probe is gadolinium-ethoxybenzyl-diethylenetriamine-pentaacetatae (Gd-EOB-DTPA; Primovist), which is a clinically approved liver-targeted contrast agent that is transported into hepatocytes primarily through organic anion transporting polypeptide (OATP) 1B1 and 1B3 that can facilitate lesion identification. Motivated by work by Dr. Kevin Brindle’s lab using the rat derived OATP1A1 reporter [1], our group has shown that engineering cells to express human derived OATP1B1 or 1B3 increases their detectability on T1-weighted images upon administration of Primovist [2]. Recent concerns over gadolinium deposition in patients with renal impairments [3], has shifted the focus in the field to the development of new agents containing alternative paramagnetic ions, such as manganese. This work describes the development and characterization of a novel class of easily synthesized and easily modified amphiphilic manganese agents (arbitrarily named Mn 1 – 5), through in vitro measurements using human OATP isoforms expressed in MDA-MB-231 cancer cells, and in vivo by measuring agent uptake through endogenous OATP in healthy BALB/c mice.Methods
Human breast cancer (MDA-MB-231) cells were engineered using a lentiviral vector expressing fluorescent zsGreen to facilitate cell sorting, and either the human OATP1B1 or OATP1B3. In vitro agent characterization: Agent relaxivity (r1) was measured at low field (0.23 mT – 1 T) using fast-field cycling relaxometry, and at our imaging field strength (3 T) using a fast-spin echo inversion-recovery sequence (FSE-IR) at 37ºC. Naïve and engineered (containing one of the two OATP isoforms noted above) MDA-MB-231 cells were incubated with one of the novel manganese-agents (1.6 mM) for 90 minutes, then washed and collected for analysis. An FSE-IR sequence was used to acquire R1 maps of cell pellets to assess agent uptake through OATP. Based on the in vitro uptake data and superior r1 relaxivity at our imaging field strength of 3 T , Mn-2, Mn-3, and Mn-5 were identified as the best candidates for use in vivo. In vivo MRI: Healthy BALB/c mice (Charles River Laboratories) were imaged using a custom-built whole-body linear birdcage coil at 3 T. Animals were anaesthetized using isoflurane, catheterized through the tail vein, and placed on a temperature-controlled bed for imaging. Pre-contrast images were taken before agent administration using a 3D spoiled gradient recalled steady state acquisition (3D-SPGR). A 0.1 mmol/kg dose of agent was administered intravenously before dynamic T1-weighted imaging of agent biodistribution for 70 minutes (image acquisition every 2.3 minutes). Contrast enhancement was then compared between the pre- and post-contrast image datasets.Results
The relaxivity of the five manganese agents at 3 T ranged from 2.27 to 3.04 mM-1s-1 in PBS. In vitro uptake assays through human transporters showed significantly increased relaxation rates post incubation with the OATP targeted manganese agents (p < 0.05). In vivo MRI showed mixed renal/hepatobiliary clearance of agents, with peak contrast enhancement in the liver reaching ~200% increase from baseline. There was no statistically significant difference in the maximum contrast enhancement generated in the livers of mice receiving Mn-2, Mn-3, or Mn-5, though the duration of maximum enhancement did vary across the three groups. The dynamic imaging data across the 70-minute interval was used to assess in vivo biodistribution and pharmacokinetics by assessing the percentage contrast enhancement within a region over time. Agent washout times were not significantly different from one another in the liver, though Mn-2 did display significantly longer washout half-life in the blood compared to Mn-3 and Mn-5.Conclusions
This novel class of Mn-derived contrast agents display promising characteristics and offer a potential solution to reduce Gd-specific concerns that are associated with commercially available agents. In addition, they are prepared using a commercially available chelator and can be easily modified to express targeting moieties, opening the door for many other targeting applications.Acknowledgements
No acknowledgement found.References
1) Patrick P, et al. Dual-modality gene reporter for in vivo imaging. Proceedings of the National Academy of Sciences, 2014.
2) Nyström N et al. Longitudinal Visualization of Viable Cancer Cell Intratumoral Distribution in Mouse Models Using Oatp1a1-Enhanced Magnetic Resonance Imaging. Invest Radiol, 2019.
3) Grobner T. Gadolinium-a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant, 2006.
Figures
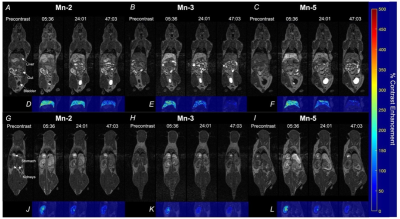
Coronal T1-weighted images through the liver are shown at representative timepoints pre- and post- administration of 0.1 mmol/kg (A) Mn-2, (B) Mn-3, (C) Mn-5. Voxel-by-voxel contrast enhancement map of whole liver sections post Mn-2 (D), Mn-3 (E), or Mn-5 (F), colour bar represents percentage contrast enhancement of a voxel. Coronal slices through the kidney are shown pre- and post- administration of 0.1 mmol/kg (G) Mn-2, (H) Mn-3, (I) Mn-5. Contrast enhancement map of whole kidney sections post Mn-2 (J), Mn-3 (K), or Mn-5 (L).
DOI: https://doi.org/10.58530/2023/4876