4873
Ultra-high Moment Gold-coated Iron Particles Allows for Longitudinal Time-lapse MRI of Individual Stem Cells in the Brain
Nikorn Pothayee1, Stephen Dodd1, Li Liu1, Gary Zabow2, and Alan Koretsky1
1National Institutes of Health, Bethesda, MD, United States, 2National Institute of Standards and Technology, Boulder, CO, United States
1National Institutes of Health, Bethesda, MD, United States, 2National Institute of Standards and Technology, Boulder, CO, United States
Synopsis
Keywords: Contrast Agent, Cell Tracking & Reporter Genes
In this study, gold coated microfabricated gold-coated iron particles are used for in vitro and in vivo labeling of immune cell and neural precursors, respectively for MRI. These particles have a pure iron core and protective layer of gold and exhibit extremely high magnetic moment relative to more commonly used, chemically synthesized, iron-oxide based particles. Following in situ labeling of neural precursor cells, we show that the migration of a large pool of individual cells can be visualized in time-lapse and longitudinal MRI.INTRODUCTION
Time-lapse MRI has been demonstrated to be useful for imaging the movement of individual immune cells in the brain of rodents [1,2]. The techniques rely on labeling of phagocytic cells through a large dose of systemic injection of superparamagnetic iron oxide nanoparticles (SPIOs), which require a large quantity of particles to be uptaken for detection of single cells. By contrast, larger iron oxide particles, in particular micron-sized iron oxide particles (MPIOs), have enabled MRI tracking of single cells but with far fewer particles required [3]. However, signal-to-noise levels and variation of iron oxide content still limit the potential range of applications. To overcome this issue, top-down microfabrication has been implemented to produce uniform magnetic particles with extremely high moment and greater detectability than MPIOs [4]. Here, we demonstrate that microfabricated gold-coated iron particles can be used to label immune cells in vitro and neural precursor cells in vivo and enable real-time tracking of individual cells in the brain of live animals. High sensitivity of the microfabricated contrast agent has enabled us to track a large pool of precursor cells through time-lapse MRI and uncover a previously unobserved migration pattern of neural precursor cells in the rodent brain.METHODS
Gold-coated iron particles were microfabricated onto a silicon wafer in a similar manner to a previously reported method [4]. The particles were subsequently removed from the wafer and embedded in a solid non-toxic, sugar-based material for storage. The particles can be resuspended by dissolving the sugar material in water and recovered by low-speed centrifugation. Mouse monocytes were labeled with varying ratio of particles to cells. MRI labeled cells suspended in agarose gel were imaged using T2*-weighted gradient-recalled echo (GRE) sequence. Parameters: isotropic resolution = 50 um, TE/TR = 12/30 ms, FA = 15°. For in vivo MRI labeling, the particles are washed and suspended in saline for injection into the lateral ventricle near the subventricular zone (SVZ) niche of the rat brain. MRI was performed at various timepoints post-injection. Parameters: isotropic resolution a 75 um, TE/TR = 10/30 ms, scan time = 20 min. The rats were given 6 mL of warm saline subcutaneously prior to imaging. Isoflurane level was maintained at 1.5 to 2% over the imaging (~ 6h). All MRI experiments were performed at 11.7 T on a Bruker Biospec MRI system (Bruker BioSpin).RESULTS AND DISCUSSION
The gold-coated iron particles were fabricated with uniform sizes and magnetic moments (Figure 1A). These particles have high moment and caused signal drops greater than 80% (Figure 1B). Elemental analysis shows the iron core and gold shell structure (Figure 1C). The particles were further modified with fluorescence probe AlexaFlour488 or 647 through polyethylene glycol linkers. Antibodies can be attached to particles using streptavidin-biotin conjugation. (Figure 2A). The modified particles were used to label mouse monocytes. The particles were taken up and mostly exhibit intracellular localization as measured by flow cytometry, Prussian blue staining, and electron microscopy (Figure 2B-E). MRI phantom imaging of suspended cells at 50 um isotropic resolution show that single cells are readily detectable (Figure 2F). In addition to in vitro labeling, it has been demonstrated that in vivo injection of iron oxide particles can label cells in vivo most widely used to image macrophages [5] or new neurons in the rodent brain [6]. We tested if the microfabricated gold coated iron particles could label neural precursors in vivo in the rodent brain. Injection into the SVZ of young adult rats allowed for visualization of the entire migration pathway along the RMS into the olfactory bulb (OB) (Figure 3A-B). These particles were internalized by the ependymal cell and neural precursor cells along the SVZ (Figure 3C). Immuno-staining with doublecortin (DCX), a marker for migrating neural precursor cells, confirmed the uptake of the particles into the cells (Figure 3C). Immuno-staining with NeuN also confirmed presence of the particles in the superficial layer of the OB, suggesting those labeled precursor cells could still migrate radially into their final location (Figure 3D). To demonstrate applicability of the microfabricated particles to track individual migrating cells, continuous MRI was performed to obtain time-lapsed video that captures real-time movement of the individual cells (Figure 4A). Most cells show movement in multiple directions with speed of ~ 100 um/h. Interestingly, some individual cells move forward and then backward (Figure 4B). To our knowledge, in vivo observation of such behavior has not been previously reported. Overall, the microfabricated particles have enabled robust tracking of migration dynamics of neural precursor cells in the rodent brain in real time (Figure 4C).CONCLUSION
We show that microfabricated iron particles with ultrahigh moment can be used for in vitro and in vivo cell tracking experiments in the rat brain. The particles are superior to the widely used MPIOs in terms of sensitivity, allowing robust real-time MRI visualization of the migration of individual cells. A previously unreported pattern of backward migration was observed which may relate to the ability of regulation of the number and time course of cells arriving to the bulb in the OB [7]. This ability to robustly measure the dynamics of migration at the single-cell level may enable details of cells migratory behavior throughout the rodent brain.Acknowledgements
This research was supported by the intramural program of the National Institute of Neurological Disordersand Stroke (NINDS), National Institutes of Health (NIH).References
[1] Mori, Y., Chen, T., Fujisawa, T. et al. Sci Rep, 4, 6997 (2014).[2] Masthoff, M., Gran, S., Zhang, X. et al. Sci Rep, 8, 9563 (2018).[3] Shapiro E. M., Skrtic S., Sharer K. et al., PNAS, 101, 10901-10906 (2004).[4] Zabow G., Dodd S. J., Shapiro E. M. et al., Magn. Reson. Med., 65, 645-655 (2011).[5] Wu Y. L., Ye Q., Foley L. M., et al. PNAS, 103, 1852-1857 (2006).[6] Sumner J. P., Shapiro E. M., Maric D., et al. Neuroimage, 44, 671-678 (2009).[7] Pothayee P., Cummings D. M., Schoenfeld T. J., Dodd S., Cameron H. A., Belluscio L., Koretsky A. P., NeuroImage, 158, 232-241 (2017).Figures
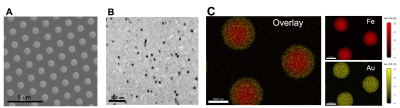
Figure 1. Fabrication and characterizations of gold coated iron particles. (A) SEM image of particles on the wafer. (B) MRI image of particles suspended in agarose gel obtained at 11.7T and 50-mm isotropic resolution. (C) Elemental analysis indicates core shell structure with gold (yellow) shell and iron (red) core.
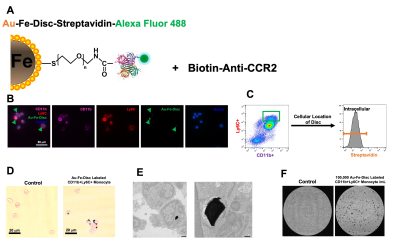
Figure 2. Application of Au-Fe-Disc for labeling CD11b+Ly6C+ classical monocytes. (A) Conjugation scheme of Au-Fe-Disc-Streptavidin-488-Biotin-CCR2. (B) Fluorescence images of labeled monocytes. (C) FACS of Au-Fe-Disc-Streptavidin-488-Biotin-CCR2 labeled CD11b+Ly6C+ monocytes. Anti-streptavidin-PE was used to identify the cellular location of the disc. (D) Prussian blue iron staining of labeled monocytes. (E) Electron micrograph of particles inside the cell. (F)MR detection of labeled monocytes.
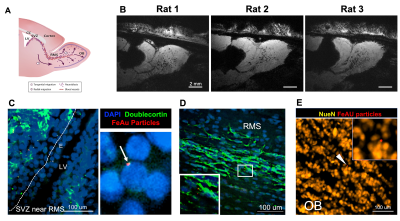
Figure 3. In vivo cell labeling and tracking of neural precursor cells. (A) Illustrative diagram of migration cell migration pathway from the SZV into RMS and OB. (B) In vivo MR images from different rats at approximately one week post-injection of FeAu particles. (C) Fluorescent image from SVZ near the RMS show an uptake of particles (red) into cells in the ependymal layer. (D). Presence of particles in the RMS closely associated with migrating precursor cells (green). (E) Presence of particles in the outer layer of the OB with neuronal nuclei (NeuN).
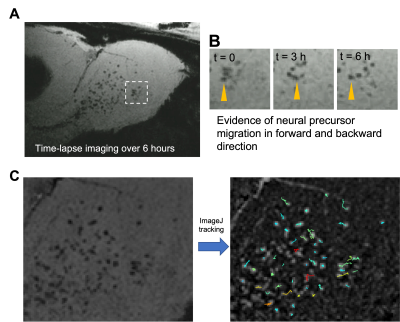
Figure 4. In vivo time-lapse imaging. (A) Time-lapse video over the course of 6 hours. (B) Example of clusters of cells that were moving in different direction including backward migration. (C) Migration patterns of individual cells. The original image was subtracted from a median-filtered image to make the particles bright. Trackmate plug-in in ImageJ was used for tracking.
DOI: https://doi.org/10.58530/2023/4873