4860
Cardiac magnetic resonance imaging with feature tracking to evaluate left atrial strain for patients with systemic lupus erythematosus1Jinling Hospital, Medical School of Nanjing University, nanjing, China, 2MR Research, GE Healthcare, beijing, China
Synopsis
Keywords: Cardiomyopathy, MR Value
This study aimed to detect left atrial (LA) strain and left ventricular (LV) stain by cardiac magnetic resonance imaging feature tracking (CMR-FT) in patients with systemic lupus erythematosus (SLE). SLE patients showed lower LVEF and multiple LV strain data than control group. Furthermore, significantly different LA reservoir, conduit and bump function were found in SLE patients than healthy controls. With these findings, CMR-FT has proven as an effective method in the diagnosis of cardiac injury in SLE patients.
Background
The systemic lupus erythematosus (SLE) is a chronic autoimmune disease which can promote the production of autoantibodies, trigger the formation and deposition of immune complexes, damages to various organs and tissues throughout the body (1). The spectrum of cardiovascular manifestations associated with SLE is considerably broad, and can directly affect the myocardium, cardiac valves, the pericardium, the conduction system, and the vasculature (2). Despite being one of the leading causes of death in patients with SLE, myocardial involvement is often asymptomatic and, thus, difficult to be identified in its early stages.Cardiac magnetic resonance (CMR) is emerging as a robust and powerful noninvasive imaging modality to evaluate myocardial involvement (3). LA strain and LV assessed by CMR feature tracking (CMR-FT) has been used in many cardiovascular diseases (4-5) and enhanced the diagnostic value which might be higher and more sensitive than conventional volumetric parameters. With these features, we assumed that CMR-FT might also have potential in quantitatively assessing myocadiac involvement for SLE patients and aimed to investigate this in this study.
Methods
SubjectsConsecutive 64 SLE patients (35.0 ± 12.3 years, 49 females) were retrospectively analyzed as well as 50 age- and gender-matched healthy controls (32.1 ± 11.2 years, 32 females). Patients with SLE were further classified into two subgroups according to mitral regurgitation: mitral regurgitation subgroup (n=32) and non-mitral regurgitation subgroup (n=32). All subjects underwent CMR examination, and patients with SLE underwent laboratory testing.
CMR protocols
All MRI examinations were performed on a 3.0 Tesla scanner (Discovery MR 750; GE Medical System, Milwaukee, WI, USA) and an 8-channel phased-array body coil and vectorcardiogram synchronization. Short-axis and Long-axis (2-, 3- and 4-chamber) cine images were acquired using a steady-state free precession end-expiratory breath-hold sequence (typical parameters: Time Repetition (TR) 41.3ms; Time Echo (TE) 1.5ms; flip angle 60°, slice thickness 8 mm; matrix size 146*256; field of view (FOV) 30cm*30cm.
Data analysis
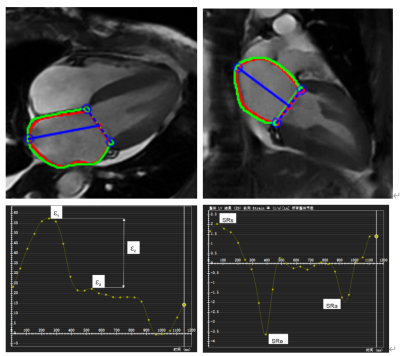
CMR studies were transferred to an offline workstation using the commercial post-processing software CVI42. LA volumetric parameters and LV volumetric parameters were averaged over 2- and 4-chamber long axis cine images and short axis cine images. Endocardial and epicardial borders of the LA and LV were manually delineated in 2- and 4-chamber long axis cine views at LA end-diastole, and then the curve of strain and strain rate changing with cardiac cycle were generated automatically (Figure 1).
Statistical analysis
Software (SPSS version 26.0; SPSS, Chicago) was used to analyze demographic data. Continuous variables with normal distribution were presented as mean ± SD, whereas continuous variables without normal distribution were presented as median (interquartile range). Categorical variables were presented as numbers and percentages. Comparison between control group and SLE group was performed by independent, two-sample t-test with normal distribution, Mann-Whitney U test for non-normally distributed data. For comparison among the three groups (control group, SLE group with mitral regurgitation and SLE group without mitral regurgitation), analysis of variance (ANOVA) test was performed for normally distributed data followed by least significant difference (LSD) multiple comparisons for homogeneity of variance and Tamhane’s T2 post-hoc comparison for heterogeneity of variance; Kruska-Wallis test followed by Bonferroni’s post-hoc comparison for non-normally distributed data. Chi-squared test for categorical variables.
Receiver-operating characteristic (ROC) curves were constructed, and areas under the curves were measured to determine cut-off values of strain for optimal sensitivity and specificity. Test-retest reproducibility and intra/interobserver variability were assessed using the Bland-Altman method to test the limits of agreement, two-way mixed effect intraclass correlations (ICC) calculated for absolute agreement. For any statistical comparison, a p value < 0.05 was considered as statistically significant.
Results
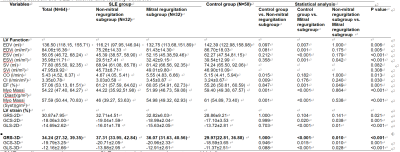
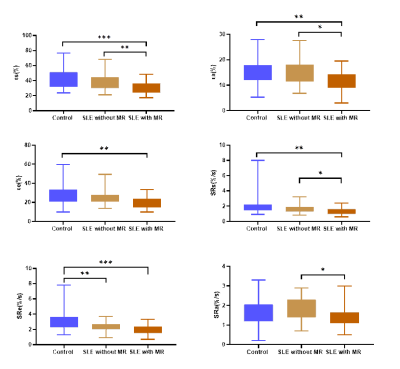
Patients in both the mitral regurgitation and non-mitral regurgitation subgroup showed higher LA volume and LV volume than control subjects (p < 0.05). SLE patients showed lower LVEF and LV strain data than control group (p < 0.05).Also, abnormal LA reservoir, conduit and bump function were observed in SLE patients, with more sever reservoir and bump function in mitral regurgitation subgroup (p < 0.05) (Table 1, 2) (Figure 2).In the SLE group, bump strain correlated positively with age, course and SLA-DAI, correlated inversely with urinary RBC count (p < 0.05).
Conclusions
CMR-FT based LV and LA performance could be further explored as a diagnostic tool to evaluate LA and LV function in SLE patients.Acknowledgements
No acknowledgement found.References
1. Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 2010; 39:257-268.
2. Feng J, Zhai Z, Wang Z, et al. Speckle tracking imaging combined with myocardial comprehensive index to evaluate left ventricular function changes in patients with systemic lupus erythematosus. Echocardiography 2021; 38:1632-1640.
3. Evin M, Cluzel P, Lamy J, et al. Assessment of left atrial function by MRI myocardial feature tracking. J Magn Reson Imaging 2015; 42:379-389.
4. Backhaus SJ, Kowallick JT, Stiermaier T, et al. Cardiac magnetic resonance myocardial feature tracking for optimized risk assessment after acute myocardial infarction in patients with type 2 diabetes. Diabetes 2020; 69:1540-1548.
5. Doerner J, Bunck AC, Michels G, Maintz D, Baessler B. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol 2018; 104:120-128.
Figures