4847
A clinical application of deep learning reconstructed LGE combined with coronary calcification scoring in coronary artery disease1Department of Radiology, Renmin Hospital of Wuhan University, Wuhan, China, 2GE Healthcare, MR Research China, Beijing, China, 3General Electric Medical (China) Co, Beijing, China
Synopsis
Keywords: Myocardium, Machine Learning/Artificial Intelligence, late gadolinum enhancement
Preclinical disease is primarily assessed through the coronary artery calcium score (CACS) and used for risk assessment, screening CACS is a reliable indicator for the assessment of coronary artery disease in our study, FWHM analysis of PSMDEDL and PSMDEO showed moderate correlation between the percentage of enhancement area and CACS, beneficial for check-up with less imaging time and low radiation screening. This finding should be further validated in a larger sample size. Moreover, threshold techniques such as 2SD to 5SD were sensitive to signal intensity and should be concerned for analysis on deep-learning reconstructed images, especially missing detection rate.Introduction
Coronary artery calcium scoring (CACS) is a strong independent predictor of cardiovascular events [1]. Time-saving artificial intelligence (AI)-based CACS is well correlated with manual CACS in terms of risk categories [2-4]. A study reported the prevalence of unrecognized myocardial infarction (UMI) increases with increasing burden of coronary artery calcium (CAC) but late gadolinium enhancement only showed UMI in 23 of 872 subjects (2.64%) [2, 5-7]. Detection rate of late gadolinium enhancement (LGE) is important while increased spatial resolution prolong scan time and higher possibility of failure to imaging [8]. LGE acquired with deep learning reconstruction (DLR) technique phase-sensitive delayed myocardial enhancement (PSMDE) may improve image quality and disease diagnosis efficacy on coronary artery disease (CAD) induced myocardial injury [1,9]. In this study, we investigated different quantitation methods to detect presence of scar on PSMDE images, and attempted to dig out whether DLR-based PSMDE can better quantify calcification in CAD patients and show good agreement with AI-CACS.Materials and methods
This study was approved by our hospital (Approval No. 2022K-K083). A total of 51 CAD patients underwent routine cardiac MRI scans with the short-axis cardiac scans of the PSMDE sequence [conventional image reconstruction (PSMDEO) and commercial AIRTM Recon DL (PSMDEDL)] on 3.0 T MRI scanner (Signa Architect, GE Healthcare) at our hospital from April to September 2022. Parameters of PSMDE were shown in Table 1. Fifteen minutes ahead of scanning the PSMDEO and PSMDEDL sequences, gabexidine glucosamine injection (Modic, Shanghai Boris Pharmaceutical Co., Ltd.) was administered at 0.1 mmol/kg body weight at a flow rate of 3.5 ms/s and an equivalent amount of saline at the same flow rate.40 of 51 patients underwent a non-enhanced chest CT scan 1 day prior to the CMR examination (Xtream Edition, GE Healthcare, 256Rows). The Agatston score for left anterior descending coronary artery (LAD Agatston score), left circumflex coronary artery (LCX Agatston score) and right coronary artery (RCA Agatston score) and a sum score of abovementioned arteries (total Agatston score) of the non-gated chest CT flat-scan was obtained using the AI-CACS software from Shukun Technology (Fig.3a.).
Myocardial signal intensity (SI) was obtained based on S1-S16 segments using threshold techniques with different times of standard deviation (2SD, 3SD, 4SD, 5SD) above remote myocardium and with full width at half maximum (FWHM) method in PSMDEO and PSMDEDL image sets. The enhancement area (mass) and percentage of enhancement area (Parea) of the whole heart were calculated and differences of each two were compared (Fig.1,2.). Pearson correlation analysis for the correlation between the percentage of enhancement areas and the Agatston score of the CACS was conducted using SPSS (version 25.0, Chicago, IL). P<0.05 was considered statistical difference.
Results
For 2SD, 3SD, 4SD and 5SD methods, higher SI values in the whole heart, the basal, middle and apical segments (Table 2) and percentage showed on PSMDEDL than on PSMDEO (all P < 0.05). For the FWHM method, the higher SI values in the whole heart on PSMDEDL than on PSMDEO (P < 0.05) but not in the basal, middle and apical segments myocardium (P > 0.05). Moreover, there were no statistically significant differences of mass (Z=0.67, P=0.50) and Parea (Z=0.76, P=0.45) between PSMDEDL and on PSMDEO. The percentage of total enhancement area on both PSMDEDL and PSMDEO images using FWHM was correlated with the total Agatston score(Fig.3b.) (rDL=0.40, rO=0.46, P < 0.05).Discussion and conclusions
SI were significantly different between quantitative methods, indicating that tiny alteration on images greatly affect quantitative diagnosis. Convolutional neural network (CNN)-based image reconstruction is a very effective tool in noise suppression and may reduce variance of SI in the distal myocardium [10]. Consistent with previous studies, the FWHM method showed higher consistency between PSMDEO and PSMDEDL in our study, for it is the least sensitive to noise levels [10-14]. In contrast, the sensitivity to abnormal myocardium of the SD method increased, for enhancement quantification of the SD method mainly depends on the mean and SD of SI within ROIs in the distal myocardium.The fair correlation between the percentage on PSMDEDL and PSMDEO sequences and CACS suggested that PSMDE was highly efficacious in detecting myocardial infarction in patients with CAD. It has been shown that the combination of CACS and myocardial perfusion imaging (MPI) can improve the diagnostic accuracy of obstructive CAD. That is, CACS reflects CAD burden in epicardial coronary arteries and PSMDE can reflect myocardial blood supply through vascular endothelial function [1], this finding of good agreement between PSMDE and CACS would benefit patients and doctors for check-up on MRI.
Analysis on PSMDEDL images should be concerned using threshold techniques, including 2SD to 5SD, for its sensitivity of signal intensity and there was significant difference between PSMDEDL and PSMDEO in spite that it is generally believed that the Signal Threshold versus Reference Mean (STRM) ≧ 3SD provide the best reference for general use. In our study, PSMDEDL images showed good image quality with less noise and reliable results using FWHM analysis, indicating the FWHM technique was independent of the image quality. The finding of Parea significantly correlated with CACS should be further validated.
Acknowledgements
No acknowledgement found.References
[1] Attila Feher, Konrad Pieszko, Robert Miller, et al. Integration of coronary artery calcium scoring from CT attenuation scans by machine learningimproves prediction of adverse cardiovascular events in patients undergoing SPECT/CT myocardial perfusion imaging. Journal of Nuclear Cardiology, published online: 04 October 2022. DOI: 10.1007/s12350-022-03099-x.
[2] Rijlaarsdam-Hermsen D, Lo-Kioeng-Shioe M, van Domburg RT, et al. Stress-Only Adenosine CMR Improves Diagnostic Yield in Stable Symptomatic Patients with Coronary Artery Calcium. JACC Cardiovasc Imaging, 2020,13(5):1152-1160. DOI: 10.1016/j.jcmg.2019.12.009.
[3] Bavishi C, Argulian E, Chatterjee S, Rozanski A. CACS and the frequency of stress-induced myocardial ischemia during MPI: a meta-analysis. J Am Coll Cardiol Img, 2016,9(5):580–589. DOI:10.1016/j.jcmg.2015.11.023.
[4] Xu J, Liu J, Guo N, et al. Performance of artificial intelligence-based coronary artery calcium scoring in non-gated chest CT. Eur Radiol, 2021,145(12):1-11. DOI: 10.1016/j.ejrad.2021.110034.
[5] Liu B, Dardeer AM, Moody WE, et al. Reference ranges for three-dimensional feature tracking cardiac magnetic resonance: comparison with twodimensional methodology and relevance of age and gender. Int J Cardiovasc Imaging, 2018, 34(1):761-775. DOI: 10.1007/s10554-017-1277-x.
[6] Dastidar AG, Baritussio A, De Garate E, et al. Prognostic role of CMR and conventional risk factors in myocardial infarction with nonobstructed coronary arteries. JACC Cardiovasc Imaging, 2019,12(10):1973–1982. DOI: 10.1016/j.jcmg.2018.12.023.
[7] Pesapane F, Codari M, Sardanelli F, et al. Artificial intelligence in medical imaging: threat or opportunity? Radiologists again at the forefront of innovation in medicine. Eur Radiol Exp, 2018,2(1):1-10. DOI: 10.1186/s41747-018-0061-6.
[8] Min Jae Cha, Sung Mok Kim, Yiseul Kim, et al.Unrecognized myocardial infarction detected on cardiac magnetic resonance imaging: Association with coronary artery calcium score and cardiovascular risk prediction scores in asymptomatic Asian cohort. PLoS One, 2018,13(9): e0204040. DOI: 10.1371/journal.pone.0204040. eCollection 2018.
[9] Nikki van der Velde,H. Carlijne Hassing,Brendan J. Bakker, et al. Improvement of late gadolinium enhancement image quality using a deep learning–based reconstruction algorithm and its influence on myocardial scar quantification. Eur Radiol,2021,31(6):3846–3855. DOI:10.1007/s00330-020-07461-w.
[10] Silvia Pradella, Lorenzo Nicola Mazzoni, Mayla Letteriello, et al. FLORA software: semi-automatic LGE-CMR analysis tool for cardiac lesions identification and characterization. Radiol Med, 2022,127(6):589-601. DOI: 10.1007/s11547-022-01491-8.
[11] Bustin A, Janich MA, Brau AC, et al. Joint denoising and motion correction: initial application in single-shot cardiac MRI[J]. J Cardiovasc Magn Reson, 2015,17(Suppl 1): Q29.
[12] Yoko Mikami, Louis Kolman, Sebastien X Joncas, et al. Accuracy and reproducibility of semi-automated late gadolinium enhancement quantification techniques in patients with hypertrophic cardiomyopathy[J]. Journal of Cardiovascular Magnetic Resonance, 2014, 16(85) :1-9. DOI:10.1186/s12968-014-0085-x.
[13] Emmanuelle Vermes, Helene Childs, Iacopo Carbone, et al. Auto-Threshold Quantification of Late Gadolinium Enhancement in Patients with Acute Heart Disease[J]. J Magn Reson Imaging, 2013,37(2):1-9. DOI: 10.1002/jmri.23814.
[14] Muscogiuri G, Martini C, Gatti M, et al. Feasibility of late gadolinium enhancement (LGE) in ischemic cardiomyopathy using 2D-multisegment LGE combined with artificial intelligence reconstruction deep learning noise reduction algorithm[J]. Int J Cardiol,2021,369(12):164-170. DOI:10.1016/j.ijcard.2021.09.012.
Figures
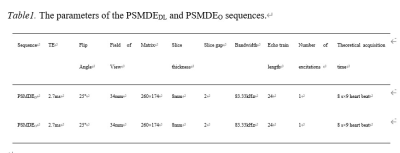
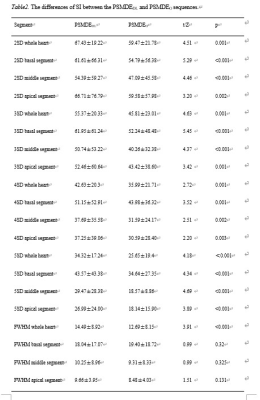
Table2. The differences of SI between the PSMDEDL and PSMDEO sequences.
Note: SI, signal intensity.
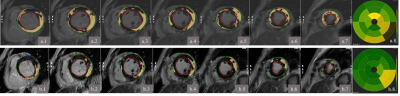
Figure 1. Schematic diagram of quantitative delineation on PSMDE sequence. a. PSMDEDL sequence, b. PSMDEO sequence. 1-7. Delineated left ventricular endomyocardium (pink circle < large >), outer membrane (green circle); 8. Schematic diagram of SI values corresponding to 16 segments of left ventricular myocardial enhancement.
Note: SI, signal intensity.
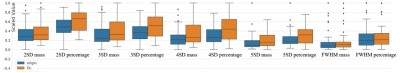
Figure 2. The differences of enhancement area (mass) and percentage of enhancement area (Parea) between the PSMDEDL and PSMDEO sequences using different threshold and FWHM methods. The vertical coordinate indicates the normalized value.
Note: FWHM, full width at half maximum.
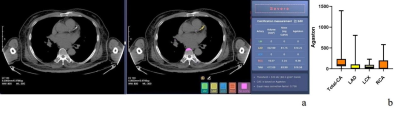
Figure 3a. Schematic diagram of Agatston score obtained by AI-CACS software on ungated non-enhanced chest. 3b. The Agaston of the CACS. Total Agatston score, LAD Agatston score, LCX Agatston score and RCA Agatston score was 97.23, 8.45, 42.08, 0.98, respectively.
Note: AI, artificial intelligence. CACS, coronary artery calcification score. Total-Agatston, sum score of coronary arteries. LAD-Agatston score, Agatston score for left anterior descending coronary artery. LCX-Agatston, left circumflex coronary artery. RCA-Agatston, right coronary artery.