4846
1H NMR of downfield metabolites in swine heart after myocardial infarction
Sophia Swago1, Jacob Smothers2, Neil E. Wilson3, Mark A. Elliott3, Ravi Prakash Reddy Nanga3, Robert Gorman4, Cory Tschabrunn5, Arjun Sengupta6, Aalim Weljie6, Ravinder Reddy3, and Walter R. Witschey3
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Chemistry, West Virginia University, Morgantown, WV, United States, 3Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4Department of Surgery, University of Pennsylvania, Philadelphia, PA, United States, 5Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States, 2Department of Chemistry, West Virginia University, Morgantown, WV, United States, 3Department of Radiology, University of Pennsylvania, Philadelphia, PA, United States, 4Department of Surgery, University of Pennsylvania, Philadelphia, PA, United States, 5Department of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 6School of Medicine, University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Myocardium, Spectroscopy
Growing interest into in vivo 1H MRS of downfield metabolites (>4.7 ppm) has necessitated greater understanding of the metabolite contributions of peaks found in the downfield. 1H NMR provides high-SNR, high-spectral resolution data that can aid in the identification of metabolite resonances. We collected downfield NMR spectra at 700 MHz from infarct and remote myocardium extract of pig hearts after myocardial infarction. We were able to assign peaks to NAD+, NADH, ATP, and AMP in the downfield region and quantified these metabolites in infarct and remote myocardium.Introduction
While conventional magnetic resonance spectroscopy has been used for decades to investigate metabolites resonating upfield of water (>4.7 ppm), there has been growing interest in detecting downfield metabolites that resonate >4.7 ppm in vivo. The downfield region of the 1H MRS spectrum is known to include many physiologically relevant metabolites in the 8 to 10 ppm range, such as NAD+, carnosine, ATP, and tryptophan, there remain many uncertainties regarding the identification of downfield peaks(1-4). Short T2 relaxation times of downfield metabolites and overlapping peaks make it difficult to determine what metabolites contribute to the peaks observed in vivo. Ex vivo NMR of tissue extract has a high signal-to-noise ratio and high spectral resolution and is therefore able to aid in the identification of downfield metabolite peaks, as well as lend insight into changes in these metabolites in diseased tissue(5,6). In this study we present 1H NMR spectra from remote and infarcted myocardium collected from pig hearts.Methods
Ischemia-reperfusion injury (IRI) was induced in two healthy Yorkshire Swine by occlusion of the mid- left anterior descending coronary artery for 90 minutes. The animals were sacrificed 1-week post IRI, and samples collected from non-infarcted (remote) left ventricular myocardium (n=3) and from infarcted left ventricular myocardium (n=3). Samples were flash frozen in liquid nitrogen. Metabolites were extracted by addition of 200 μl of methanol, 200 μl of cholorform, and 100 μl of water per 50 mg of frozen tissue to the samples. Samples were homogenized, centrifuged, and the upper fraction removed and dried. Samples were reconstituted in 90%/10% H2O/D2O or 100% D2O. 1D 1H NMR spectra were collected on a 700 MHz spectrometer (Avance III, Bruker BioSpin. Ettlingen, Germany) with water suppression performed by excitation sculpting. Scan parameters were as follows: 78k data points, 1k scans, relaxation delay 1 second, auto calibration of 90 degree pulse, auto receiver gain. Processing was done by filtering the time-domain signal with an exponential multiplication (EM) window function with line broadening = 0.3 Hz. Phase and baseline correction was done automatically.Spectra were analyzed in Mnova 14.1.2 (Mestrelab Research). Peaks were identified using the Human Metabolome Database and previous literature(5,6). Peaks in the 8 to 10 ppm range were integrated and were referenced to an internal standard of DSS of 0.26 or 0.25 mM and corrected for number of contributing protons and normalized to grams of tissue of the sample. Averages and standard deviations of metabolite concentrations in infarct and remote myocardium are reported.
Results
In the obtained spectra, unambiguous resonances were assigned for nicotinamide adenine dinucleotide (NAD+) at 9.33, 9.15, 8.83 ppm; reduced nicotinamide adenine dinucleotide (NADH) at 8.37 ppm; adenosine monophosphate (AMP) at 8.58 ppm, and adenosine trisphosphate (ATP) at 8.52 ppm (Fig.1). Metabolite concentration is reported as uM/g of tissue, and the concentration of NAD+ is obtained from the average of the three NAD+ peaks. In the remote myocardial samples, the average ± standard deviation concentrations of metabolites were: [NAD+] = 1.80±0.39 μM/g; [AMP] = 4.91±0.69 μM/g; [ATP] = 13.35 ± 2.53 μM/g; and NADH = 0.97 ± 0.77 μM/g. In two samples taken from infarcted tissue, no metabolites were detected in the 8 to 9.5 ppm range. In one infarct sample, no NAD+ was detected, but AMP, ATP, and NADH were detected at concentrations of 0.73 μM/g, 0.57 μM/g, and 0.36 μM/g, respectively (Fig. 2, Fig. 3).Discussion and Conclusion
In these preliminary results, peaks corresponding to proton resonances originating form NAD+, ATP, AMP, and NADH were identified in the downfield 8 to 10 ppm range in pig myocardial tissue extracts. There were decreased levels of downfield metabolites resonating in the 8 to 10 ppm range in infarcted tissue harvested from a heart with ischemia-reperfusion injury. Further NMR-based metabolomics of the downfield region of the 1H spectrum will continue to aid in determining peak assignments and changes in metabolite concentrations for in vivo downfield spectroscopy.Acknowledgements
Research reported in this publication was supported by the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under award Number P41EB029460 and the National Heart, Lung, and Blood Institute under award Numbers R01HL137984 and F31HL158217.References
- Nanga RPR, Elliott MA, Swain A, Wilson N, Swago S, Soni ND, Witschey WR, Reddy R. Identification of l‐Tryptophan by down‐field 1H MRS: A precursor for brain NAD+ and serotonin syntheses. Magnetic Resonance in Medicine 2022;88(6):2371-2377.
- Swago S, Cember A, Moon B, Bagga P, Wilson N, Elliott MA, Hariharan H, Reddy R, Witschey W. Characterization of cross-relaxation in human skeletal muscle using downfield 1H MRS at 7T. 2021.
- Bagga P, Hariharan H, Wilson NE, Beer JC, Shinohara RT, Elliott MA, Baur JA, Marincola FM, Witschey WR, Haris M. Single‐Voxel 1H MR spectroscopy of cerebral nicotinamide adenine dinucleotide (NAD+) in humans at 7T using a 32‐channel volume coil. Magnetic Resonance in Medicine 2020;83(3):806-814.
- Goncalves SI, Ligneul C, Shemesh N. Short echo time relaxation-enhanced MR spectroscopy reveals broad downfield resonances. Magn Reson Med 2019;82(4):1266-1277.
- Heitzman JA, Dobratz TC, Fischer KD, Townsend D. A 1H-NMR approach to myocardial energetics. Scientific reports 2020;10(1):1-11.
- Nagana Gowda G, Abell L, Lee CF, Tian R, Raftery D. Simultaneous analysis of major coenzymes of cellular redox reactions and energy using ex vivo 1H NMR spectroscopy. Analytical chemistry 2016;88(9):4817-4824.
Figures

Figure 1. Representative downfield NMR spectra from remote myocardium (top, red) and from infarcted myocardial (bottom, blue). Three NAD+ peaks are located at 9.23 ppm, 9.04 ppm, and 8.73 ppm. AMP, ATP, and NADH have resonance at 8.49 ppm, 8.43 ppm, and 8.35 ppm, respectively.
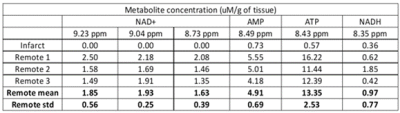
Figure 2. Metabolite assignments to downfield peaks. Concentrations of downfield metabolites in one infarct sample and means and standard deviations of metabolites in remote myocardium samples.
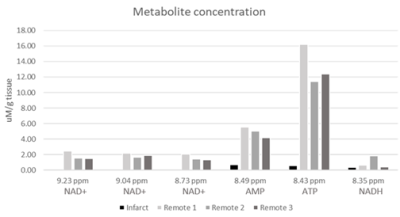
Figure 3. Downfield metabolite concentrations measured in μM/g tissue from all pig myocardium samples.
DOI: https://doi.org/10.58530/2023/4846