4843
Fractal Analysis of Left Ventricular Trabeculae in HFpEF Patients with Multivessel Coronary Artery Disease1Department of Cardiovascular Surgery, Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital, Shanghai, China, 2Philips Healthcare, Shanghai, China, 3Department of Radiology, Shanghai Jiao Tong University School of Medicine Affiliated Renji Hospital, Shanghai, China
Synopsis
Keywords: Heart, Heart, HFpEF
HFpEF patients with multivessel CAD have changes in myocardial trabecular complexity. The left ventricular FD obtained with fractal analysis can reflect the complexity of myocardial trabecular and has an independent predictive value for the diagnosis of HFpEF in patients with multivessel CAD. Including FD into the diagnostic model can help improve the diagnosis.Introduction
Ischemic coronary artery disease is one of the risk factors of heart failure (HF). Heart failure with preserved ejection fraction (HFpEF) is a form of HF whose incidence is steadily increasing every year1. HFpEF is thought to be related to multivessel coronary artery disease2, but has been rarely studied3. The diagnosis of HFpEF is challenging as it requires an evaluation of clinical history, physical examination, natriuretic peptide testing, echocardiographic data, and invasive catheterization testing to demonstrate poor cardiac output4. Endocardial trabecula is a complex myocardial network extending into two ventricles. Simulation data have shown that trabeculae affect hemodynamics and improve mechanical efficiency5,6. The varicose morphology of left ventricular trabecular network is related to hemodynamic factors. It is a variable phenotype and is associated with cardiac load7. Fractal analysis is a sensitive, automated, and highly reproducible method for detecting subtle changes in endocardial trabeculae8. With cardiac magnetic resonance short-axis cine sequences, the fractal dimension (FD) representing the complexity of the trabeculae can be calculated to determine their morphological changes. The aim of this study was to understand the complex changes in endocardial trabeculae with fractal analysis in the HFpEF patients with multivessel coronary artery disease. The results of this study provide novel imaging characteristics for disease diagnosis.Methods
Fractal analysis was performed by a custom-written code (FracAnalyse) in MATLAB (Math Works Inc.), which has been validated in several studies9. For each slice, the analysis procedure includes three steps: First, a region of interest was selected outside the LV endocardial border on short-axis cine stacks at end-diastole. Then, endocardial border was extracted using an image segmentation algorithm. Third, the FD value was calculated using a box-counting approach. Global FD was defined as an average of all FD in all measured slices. Maximal Basal FD and Maximal Apical FD were defined as the maximal value of the basal and apical slices of the ventricle. Mean Basal FD and Mean Apical FD were defined as the average values of the corresponding slices.Results
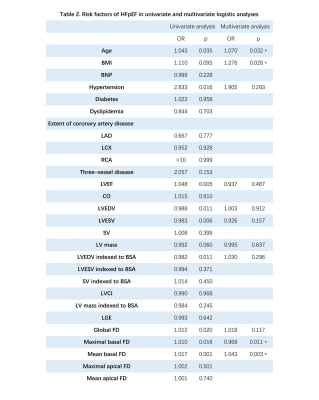
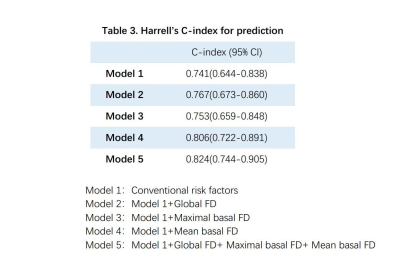
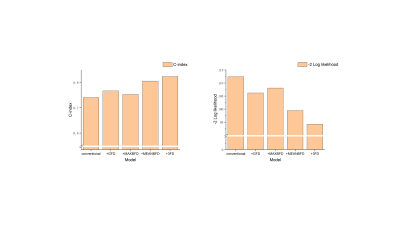
Compared to the healthy group, global FD and mean basal FD were significantly higher in HFpEF-CAD patients (p<0.05), while no difference was seen in non-HFpEF-CAD patients. Compared with non-HFpEF-CAD patients, global FD, maximal basal FD, and mean basal FD were significantly elevated in HFpEF-CAD patients (p< 0.05, Table 1). In the univariate logistic regression analysis, we included the traditional risk factors for HFpEF10, extent of coronary artery disease, CMR parameters, and FD as exposure factors (Table 2). The results of the analysis showed that global FD, maximal basal FD, and mean basal FD were significant univariate predictors, while maximal apical FD and mean apical FD were not. Significant univariate parameters were added to the multivariate logistic regression analysis. Maximal basal FD and mean basal FD were identified as significant multivariate predictors. The value of FD for diagnosing HFpEF in patients with multivessel coronary artery disease was assessed. Compared with the conventional model, incorporation of global FD, maximal basal FD, and mean basal FD into the prediction model improved the Harrell's C-index, while the simultaneous inclusion of the three FD led to the highest Harrell's C-index (Table 3). Moreover, the prediction model including FD also showed better goodness-of-fit (-2 log likelihood ratio test; p < 0.05, Figure 2). These results suggested that FD helps to improve the diagnostic model for HFpEF in patients with multivessel coronary artery disease.Discussion
The incidence of HFpEF is increasing and 4.9% of the general population over 60 years of age is diagnosed with HFpEF11. No effective treatment has been identified to date, possibly due to the pathophysiological heterogeneity within the broader clinical spectrum. Therefore, effective diagnostic methods are needed to facilitate individualized treatment4. Excessive proliferation of ventricular trabeculae has been found to be associated with multivessel CAD12-14. Given that left ventricular compensation is inevitable for the heart to maintain normal ventricular function, trabecular hyperplasia and changes in complexity will be potentially used for early diagnosis in patients with HFpEF15. Fractal analysis has been demonstrated as a reliable method to assess trabecular complexity in several studies16-18. In this study, LV global FD and mean basal FD showed significant differences in HFpEF patients, but the difference in global FD was not statistically significant in logistic regression analysis, possibly due to the major compensatory function occurring in the middle or basal of the left ventricle during maintenance of LV function. Wang19 et al. estimated diastolic myocardial stiffness and stress by personalized biomechanical modeling and analysis techniques. They found that heart failure patients had higher myofiber stress in mid-ventricular region. This is consistent with our finding. The accuracy and goodness-of-fit of the model was improved before including FD into the diagnostic model, especially for mean basal FD. The combined inclusion of three FD yielded the best diagnostic model.Conclusion
In summary, HFpEF patients with multivessel CAD have changes in myocardial trabecular complexity. The left ventricular FD obtained with fractal analysis can reflect the complexity of myocardial trabecular and has an independent predictive value for the diagnosis of HFpEF in patients with multivessel CAD. Including FD into the diagnostic model can help improve the diagnosis.Acknowledgements
We are very grateful to the Philips Healthcare team for their support in image analysis.References
1.Zhang, X. et al. A Bibliometric Analysis of Heart Failure with Preserved Ejection Fraction From 2000 to 2021. Curr Probl Cardiol, 101243, doi:10.1016/j.cpcardiol.2022.101243 (2022).
2.Hwang, S. J., Melenovsky, V. & Borlaug, B. A. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol 63, 2817-2827, doi:10.1016/j.jacc.2014.03.034 (2014).
3. Patel, M. R. et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 Appropriate Use Criteria for Coronary Revascularization in Patients With Stable Ischemic Heart Disease: A Report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 69, 2212-2241, doi:10.1016/j.jacc.2017.02.001 (2017).
4.Borlaug, B. A. Evaluation and management of heart failure with preserved ejection fraction. Nat Rev Cardiol 17, 559-573, doi:10.1038/s41569-020-0363-2 (2020).
5.Kulp, S. et al. Using high resolution cardiac CT data to model and visualize patient-specific interactions between trabeculae and blood flow. Med Image Comput Comput Assist Interv 14, 468-475, doi:10.1007/978-3-642-23623-5_59 (2011).
6.Fatemifar, F., Feldman, M., Clarke, G., Finol, E. A. & Han, H. C. Computational modeling of human left ventricle to assess the role of trabeculae carneae on the diastolic and systolic functions. J Biomech Eng, doi:10.1115/1.4043831 (2019).
7.Captur, G., Syrris, P., Obianyo, C., Limongelli, G. & Moon, J. C. Formation and Malformation of Cardiac Trabeculae: Biological Basis, Clinical Significance, and Special Yield of Magnetic Resonance Imaging in Assessment. Can J Cardiol 31, 1325-1337, doi:10.1016/j.cjca.2015.07.003 (2015).
8.Captur, G. et al. Quantification of left ventricular trabeculae using fractal analysis. J Cardiovasc Magn Reson 15, 36, doi:10.1186/1532-429X-15-36 (2013).
9.Yu, S. et al. Correlation between left ventricular fractal dimension and impaired strain assessed by cardiac MRI feature tracking in patients with left ventricular noncompaction and normal left ventricular ejection fraction. Eur Radiol 32, 2594-2603, doi:10.1007/s00330-021-08346-2 (2022).
10.Pieske, B. et al. How to diagnose heart failure with preserved ejection fraction: the HFA-PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 40, 3297-3317, doi:10.1093/eurheartj/ehz641 (2019).
11.van Riet, E. E. et al. Epidemiology of heart failure: the prevalence of heart failure and ventricular dysfunction in older adults over time. A systematic review. Eur J Heart Fail 18, 242-252, doi:10.1002/ejhf.483 (2016).
12.Patel, A. R. & Mor-Avi, V. Are trabeculae and papillary muscles an integral part of cardiac anatomy: or annoying features to exclude while tracing endocardial boundaries? JACC Cardiovasc Imaging 5, 1124-1126, doi:10.1016/j.jcmg.2012.06.008 (2012).
13.Chuang, M. L. et al. Correlation of trabeculae and papillary muscles with clinical and cardiac characteristics and impact on CMR measures of LV anatomy and function. JACC Cardiovasc Imaging 5, 1115-1123, doi:10.1016/j.jcmg.2012.05.015 (2012).
14.Captur, G. et al. Fractal Analysis of Myocardial Trabeculations in 2547 Study Participants: Multi-Ethnic Study of Atherosclerosis. Radiology 277, 707-715, doi:10.1148/radiol.2015142948 (2015).
15.Brandes, R., Maier, L. S. & Bers, D. M. Regulation of mitochondrial [NADH] by cytosolic [Ca2+] and work in trabeculae from hypertrophic and normal rat hearts. Circ Res 82, 1189-1198, doi:10.1161/01.res.82.11.1189 (1998).
16.Captur, G. et al. Community delivery of semiautomated fractal analysis tool in cardiac mr for trabecular phenotyping. J Magn Reson Imaging 46, 1082-1088, doi:10.1002/jmri.25644 (2017).
17.Wang, J. et al. Fractal Analysis: Prognostic Value of Left Ventricular Trabecular Complexity Cardiovascular MRI in Participants with Hypertrophic Cardiomyopathy. Radiology 298, 71-79, doi:10.1148/radiol.2020202261 (2021).
18.Dawes, T. J. W. et al. Fractal Analysis of Right Ventricular Trabeculae in Pulmonary Hypertension. Radiology 288, 386-395, doi:10.1148/radiol.2018172821 (2018).
19.Wang, Z. J. et al. Left Ventricular Diastolic Myocardial Stiffness and End-Diastolic Myofibre Stress in Human Heart Failure Using Personalised Biomechanical Analysis. J Cardiovasc Transl Res 11, 346-356, doi:10.1007/s12265-018-9816-y (2018).
Figures
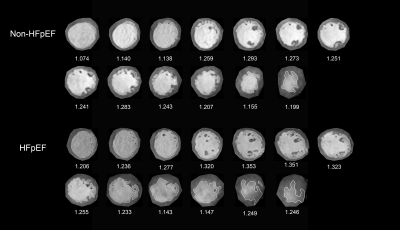
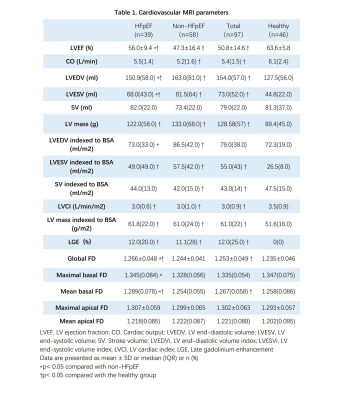
Table 1. Cardiovascular MRI parameters of the study population.