4842
New Tagging MRI Method is More Sensitive than Ejection Fraction for Assessment of Contractile Function Recovery in Infarcted Pig Hearts1Radiology, University of Washington, Seattle, WA, United States
Synopsis
Keywords: Myocardium, Heart, myocardial infarction
A new tagging method has been proved as a sensitive approach for assessment of regional deformation in the infarcted pig’s heart. The linear tags were placed in 60-degree pattern offsets and aligned with the AHA segments. The tagging analysis algorithm uses local Fourier transformation of the isolated spectral peaks. We have shown the temporal changes in circumferential strain of the infarcted segments of myocardium and faster recovery of myocardial strain followed by 60-min ischemia-reperfusion injury in comparison with 90-min. Circumferential strain was a more sensitive parameter for earlier detection of regional contractile dysfunction in comparison with ejection fraction.Introduction
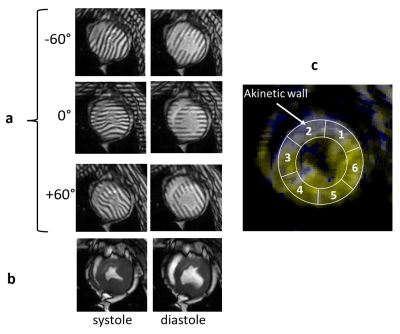
MR tagging technique is a widely accepted non-invasive method for assessment of regional myocardial deformation 1-5. Our new approach in tagging placement and analysis is based on the linear tags were placed in 60-degree pattern offsets and aligned with the AHA segments (figure 1). The tagging analysis algorithm uses local Fourier transformation of the isolated spectral peaks in SCPAMM images, which contain information about regional cardiac motion 6. The aim of the study was to test the sensitivity of this technique for detection of regional myocardial disfunction variations after different duration of myocardial infarction injury in the large animal model.Methods
Animals. 18 castrated young male Yucatan minipigs weighing between 30-40 kg were included unto the study. Myocardial infarction (MI) was modeled using percutaneous transluminal coronary artery catheterization technique for 90 minutes followed by reperfusion in 16 pigs 7. Two other pigs were subjected to a shorter period of MI equal to 60 min followed by reperfusion.CMRI acquisition. In vivo cardiac MRI studies were conducted on a 3T Ingenia CX clinical scanner (Philips, Best, Netherlands) at the different time points: on healthy animals before MI modeling and then at 2, 4, 8, and 12 weeks after MI. During the scan, the animals were sedated with a combination of Butorphanol, Acepromazine and Ketamine administered intramuscularly. Animals were then intubated and mechanically ventilated using Isoflurane and oxygen to maintain a surgical plane of anesthesia during the scan. CMRI protocol included a balanced turbo field echo (bTFE) cine sequence for assessment of the heart contractility; complimentary spatial modulation of magnetization (SCPAMM) sequence was used for tagging imaging. Tagging lines were placed in the short axis of the heart tangential to the heart wall with 60-degree shift to each other and were aligned with the standard AHA segments. The parameters of the SCPAMM sequence were the following: TR 5.8ms; TE 3.5 ms; flip angle 10 degree; field of view 350x350 mm; slice thickness 8 mm; image resolution 1.1x1.1 mm; 3 mm tag separation. All acquisitions were done with 1 signal average and breath hold.
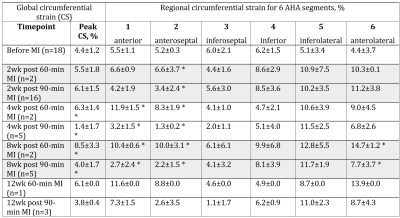
Tagging analysis. A new image processing technique for rapid analysis of tagged images uses isolated spectral peaks in SCPAMM-tagged images, which contain information about cardiac motion. Frequency is estimated by local Fourier transformation. Custom-written MATLAB software analyzes circumferential shortening in the zones of circumferential sensitivity. Measurements were combined geometrically for areas of overlap between patterns. Global as well as regional strain and strain rate in six AHA segments of one short-axis slice were measured.
Statistical Analysis. Statistical analyses were carried out in Excel software for Windows (Microsoft Inc., Redmond, WA, USA).
Results
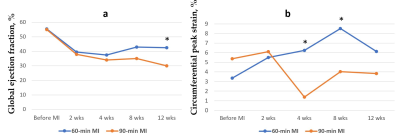
The average myocardial peak circumferential strain (CS) of the normal pig’s hearts was 4.4±1.2% (n=18). CS decreased following MI injury; changes in the global peak CS as well as regional CS in the infarcted AHA segments (anterior and anteroseptal areas) were statistically significant at 4 and 8 weeks after MI (Table 1). Global peak CS steadily increased in the pig’s heart following 60-min MI up to 8.5±3.3% at 8 weeks post-MI. Global peak CS in the 90-min MI model dropped to 1.4±1.7% by 4 weeks and stayed at the 4.0±1.7% rest of the studied time point (figure 2). In contrast with strain measurements, differences between 60- and 90-min MI models in global contractility measured by ejection fraction became significant only at 12 weeks after MI (figure 2). Regional CS was the most sensitive measure for detection of local contractility changes caused by different duration of MI: CS in the infarcted area of LV (anteroseptal AHA segment) decreased already at 2 weeks after 90-min MI (statistically significant difference with 60-min MI model).Discussion and Conclusion
We have shown temporal changes in circumferential strain of the infarcted pig’s heart following 60-and 90-min MI. Three orientations of tags at 60-degrees pattern aligned with AHA heart segments provide sensitive measure of regional contractility of the infarcted heart capable of detection in myocardial strain differences between 60-min and 90-min MI models. Circumferential strain was a more sensitive parameter for earlier detection of regional LV contractile dysfunction in comparison with global ejection fraction. Statistical significance was not reached in some of the studied time points because of the small sample size.Acknowledgements
We appreciate Lauren E. Neidig, Emily Spaulding and Gary Fye for pig surgery, help with anesthesia and breath hold technique during CMRI studies. Dr. Charles E. Murry for general support and funding.References
1. Zerhouni EA, Parish DM, Rogers WJ, Yang A, Shapiro EP. Human heart: tagging with MR imaging--a method for noninvasive assessment of myocardial motion. Radiology. 1988 Oct;169(1):59-63.
2. Axel L, Dougherty L. MR imaging of motion with spatial modulation of magnetization. Radiology 1988;169:59–63.
3. McVeigh ER, Zerhouni EA. Noninvasive measurement of transmural gradients in myocardial strain with MR imaging. Radiology 1991; 180(3): 677-83.
4. Shehata ML, Cheng S, Osman NF, Bluemke DA, Lima JA. Myocardial tissue tagging with cardiovascular magnetic resonance. J Cardiovasc Magn Reason. 2009; 11:55.
5. Collins JD. Global and regional functional assessment of ischemic heart disease with cardiac MR imaging. Radiol Clin of North Am. 2015, 53 (2):369–395.
6. Naumova AV, Kerwin W. Rapid and Robust Assessment of Circumferential Strain Recovery via Tagged MRI of Infarcted Pig Hearts After Human Cardiomyocyte Transplantation. ISMRM 2022 Proceedings. Abstract 2082.
7. McCall FC, Telukuntla KS, Karantalis V, et al. Myocardial infarction and intramyocardial injection models in swine. Nat Protoc. 2012;7(8):1479–1496.
Figures