4840
Semi-automatic scar size quantification in late gadolinium enhancement of acute to chronic myocardial infarction in a porcine model scanned at 7T
Alena Kollmann1, David Lohr1, Maya Bille1, Maxim Terekhov1, Michael Hock1, Ibrahim Elabyad1, Florian Schnitter2, Wolfgang Bauer1,2, Ulrich Hofmann2, and Laura Maria Schreiber1
1Chair of Molecular and Cellular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany, 2Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Germany
1Chair of Molecular and Cellular Imaging, Comprehensive Heart Failure Center (CHFC), University Hospital Wuerzburg, Wuerzburg, Germany, 2Department of Internal Medicine I, University Hospital Wuerzburg, Wuerzburg, Germany
Synopsis
Keywords: Myocardium, Ischemia
Late gadolinium enhancement (LGE) is considered the gold standard for the quantification of scar size. We tested in a large animal model of acute and chronic infarction if clinically used methods for the assessment of infarct size (manual planimetry and several semi-automatic approaches) in LGE images are applicable to preclinical 7T LGE imaging of porcine hearts. We found excellent intra-observer reproducibility for all methods. The tested semi-automatic methods performed differently on magnitude (MAG) and phase-sensitive inversion recovery (PSIR) images. Overall, infarct sizes measured in in vivo scans showed good correlation to ex vivo LGE measurements.Introduction
Ischemic heart disease is the leading cause of death worldwide1 and myocardial infarction (MI) as a potential complication is a common cause of heart failure. Respective pathology is increasingly assessed using cardiac magnetic resonance (CMR) and late gadolinium enhancement (LGE) is the gold standard for infarct quantification.2 In order to increase precision in quantification based on increased image resolution, CMR at ultra-high field strengths (≥7T) has become an important research modality. Due to limitations in the specific absorption rate and 7T not being CE-certified for CMR, no clinical LGE studies have been performed at 7T in humans yet. In a prior comprehensive preclinical large animal study, where such limitations did not exist and LGE imaging was thus included, we investigated the development of the LGE signal in MI from an acute to a chronic stage.3 In this study we aimed to assess the reproducibility and performance of different established methods (manual and semi-automatic approaches) of infarct size quantification on 7T LGE images of porcine hearts.Methods
The large animal study was approved by the District Government of Lower Franconia, Germany (55.2.2-2532.2-1134-16). MI was induced by 90 min balloon catheter occlusion of the left anterior descending artery in seven female German Landrace pigs. MRI measurements using a 7T MAGNETOM™ Terra system (Siemens Healthineers, Erlangen, Germany) were performed prior to MI and after infarct induction (3-4, 10-14 and ~60 days after MI). Animals were euthanized after MRI 4. LGE images were acquired using a gradient-echo sequence with TE: 3.18ms, TR: 49.52ms, and FA: optimal, and in-plane-resolutions: 0.7x0.7 mm2 (in vivo) and 0.4x0.4 mm2 (ex vivo). Both magnitude (MAG) and phase-sensitive inversion recovery (PSIR) images were generated. Post-processing of the MR images was done using the clinical software Medis Suite 3.1 (Medis Medical Imaging Systems, Leiden, the Netherlands), following clinical guidelines4. Infarct size (in percentage [%] and in mass [g]) was determined using various methods: manual planimetry and several semi-automatic methods (full width half maximum (FWHM) technique and standard deviation (n-SD) technique for 3-SD, 5-SD and 7-SD). For all methods, manual delineation of the endo- and epicardial border of the left ventricular myocardium was needed. Some images required manual correction (exclusion of artefacts or wrongly included blood pool areas). Coefficients of variability (CoV) were calculated as the standard deviation of the difference divided by the mean of two measurements. Intraclass-correlation coefficients (ICCs) were calculated and interpreted according to Koo and Li (ICC<0.5: poor, ICC=0.5-0.75: moderate, ICC=0.75-0.9: good, and ICC>0.9: excellent).5Results
Intra-observer analysis found ICCs greater than 0.9 for all methods, and CoVs ranged from 3.9% to 22.3%. Bland-Altman plots for intra-observer comparison showed excellent agreement for different methods applied to PSIR images (Fig. 1). Infarct size derived from manual planimetry showed a significant difference when using MAG or PSIR images, with MAG images underestimating the size by a mean of 3.3% and 2.8 g, respectively. The different semi-automatic methods showed varying degrees of correlation to manual analysis (see Fig. 2 and Fig. 3). For MAG images the FWHM technique was underestimating and the 3-SD technique was overestimating. 5-SD and 7-SD provided visually good infarct size estimation and were not significantly different from manual analysis. For PSIR images the performance of automatic methods varied. 7-SD clearly underestimated the infarct size, while the other tested methods showed acceptable correlation with manual planimetry (ICCs 0.70 - 0.81). For the in vivo-ex vivo comparison (Fig. 4), we found excellent correlation for PSIR images (infarct size [%]: ICC 0.94, infarct size [g]: ICC 0.93) and a moderate to excellent correlation for MAG images (infarct size [%]: ICC 0.55, infarct size [g]: ICC 0.90) due to an underestimation of infarct size [%] in the in vivo MAG scans. Bland-Altman plots for in vivo vs ex vivo comparison of scar size [% / g] derived from manual planimetry of PSIR images showed excellent agreement (Fig. 4C). For PSIR images, the difference between in vivo and ex vivo infarct size was not statistically significant (α-level of 0.05, p-values: p=0.08 for infarct size in % and p=0.2 for infarct size in g).Discussion
The data show excellent intra-observer reproducibility for all tested methods of scar size quantification. ICCs for intra-observer variability range from 0.91 to 0.99 and are in line with or higher than what has been reported in large animals [reference: ICC 0.82 - 0.96]6,7. Infarct size underestimation in MAG compared to PSIR is significant and may be due to the low blood-scar contrast in some scans, which makes it difficult to delineate endocardial border and blood pool. Since blood-scar contrast was better in ex vivo images than in in vivo images, the underestimation (in vivo MAG versus ex vivo MAG) may have similar reasons. Inferior image quality in some of the n=7 MAG in vivo scans (MRI 4) should also be considered. In addition, with n=7, the sample size in in vivo versus ex vivo comparison is rather small, which limits statistical power. We demonstrate that reproducible quantification of infarct sizes in 7T large animal studies can be achieved using both manual planimetry and selected semi-automatic methods. The threshold for semi-automatic methods should be chosen with respect to the image type (MAG or PSIR).Acknowledgements
Financial support: German Ministry of Education and Research (BMBF, grant: 01E1O1504).
L. M. Schreiber receives research support by Siemens Healthineers. The position of D. Lohr is partially funded by this research support.
Parts of this work will be used in the doctoral thesis of A. Kollmann.
References
- Tsao, C. W. et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 145, e153-e639; 10.1161/CIR.0000000000001052 (2022).
- Karamitsos, T. D., Francis, J. M., Myerson, S., Selvanayagam, J. B. & Neubauer, S. The role of cardiovascular magnetic resonance imaging in heart failure. Journal of the American College of Cardiology 54, 1407–1424; 10.1016/j.jacc.2009.04.094 (2009).
- Lohr, D. et al. Ultrahigh Field Cardiac MRI in a Large Animal Model of Acute and Chronic Infarction [Conference presentation abstract]. In Proceedings of the 2021 ISMRM & SMRT Annual Meeting & Exhibition, 15-20 May 2021, Virtual, Abstract nr. 0690.
- Schulz-Menger, J. et al. Standardized image interpretation and post-processing in cardiovascular magnetic resonance - 2020 update : Society for Cardiovascular Magnetic Resonance (SCMR): Board of Trustees Task Force on Standardized Post-Processing. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 22, 19; 10.1186/s12968-020-00610-6 (2020).
- Koo, T. K. & Li, M. Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. Journal of chiropractic medicine 15, 155–163; 10.1016/j.jcm.2016.02.012 (2016).
- Nguyen, C. et al. In vivo contrast free chronic myocardial infarction characterization using diffusion-weighted cardiovascular magnetic resonance. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance 16, 68; 10.1186/s12968-014-0068-y (2014).
- Lenkey, Z. et al. Age-independent myocardial infarct quantification by signal intensity percent infarct mapping in swine. Journal of magnetic resonance imaging : JMRI 43, 911–920; 10.1002/jmri.25046 (2016).
Figures
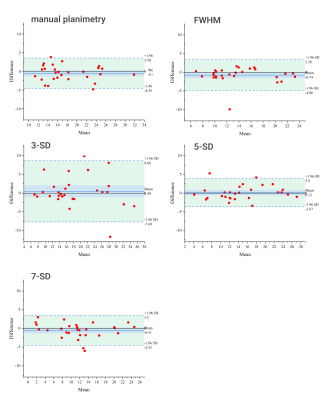
Figure 1. Bland-Altman plots for intra-observer variation of different
methods of scar size quantification based on phase-sensitive inversion recovery
(PSIR) images. Infarct size [%] has been calculated twice for each method. Methods
used are (from top left to bottom right): manual planimetry, full width half
maximum (FWHM) technique, 3 standard deviation (3-SD) method, 5 standard
deviation (5-SD) method, and 7 standard deviation (7-SD) method.

Figure 2. Different methods of scar size quantification applied to in vivo magnitude (MAG) images (top row) and phase-sensitive inversion recovery (PSIR)
images (bottom row). From left to right: manual planimetry, full width half
maximum (FWHM) technique, 3 standard deviation (3-SD) method, 5 standard
deviation (5-SD) method, and 7 standard deviation (7-SD) method. In all images,
late gadolinium enhancement (red) is present due to infarction in anteroseptal
and anterior segments of the left ventricular myocardium.
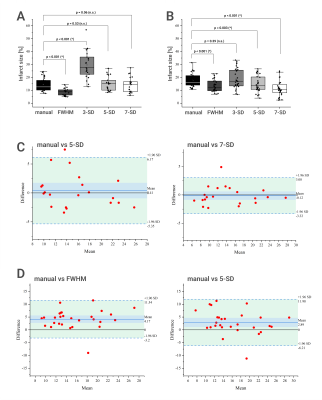
Figure 3. Comparison of different methods of scar size quantification
based on magnitude (MAG) images (A) and phase-sensitive inversion recovery (PSIR) images (B). In both diagrams, boxplots contain data for the five methods: manual
planimetry, full with half maximum (FWHM) technique, 3 standard deviation
(3-SD), 5 standard deviation (5-SD), and 7 standard deviation
(7-SD) method. P-values are given above, α-level is 0.0125. Bland-Altman plots below represent the two best performing semi-automatic
methods for MAG (C) and for
PSIR images (D) in comparison to
manual planimetry.
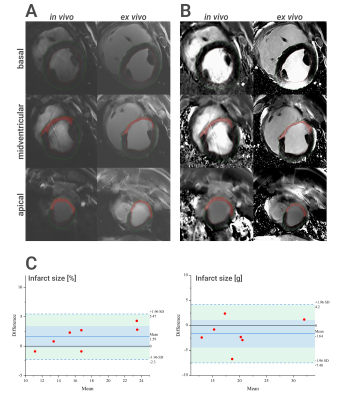
Figure 4. Comparison of in vivo and ex vivo scar size. Magnitude (MAG) images (A) and phase-sensitive inversion recovery (PSIR) images (B) of in
vivo (left) and
corresponding ex vivo images (right) for a basal, midventricular
and apical slice. The apical and midventricular slices show an enhanced (red) scar
area in anteroseptal and anterior myocardial segments (method: manual
planimetry). C: Bland-Altman plots
for comparison of in vivo vs ex
vivo infarct
size in % (left) and g (right) based on PSIR images (method: manual
planimetry).
DOI: https://doi.org/10.58530/2023/4840