4821
Identification of invasive adenocarcinoma in pulmonary ground-glass nodules using zero echo time magnetic resonance imaging (ZTE-MRI)1Department of Radiology, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China, 2MR Research China, GE Healthcare, Beijing, China, 3Philips Healthcare, Guangzhou, China
Synopsis
Keywords: Lung, Cancer
Intra-modality and inter-reader agreements of ground-glass opacity nodule (GGN) size were good between modalities (ZTE-MRI, T2WI-FS, CT). ZTE-MRI was susceptible to motion artifacts caused by breathing and vascular pulsation and had difficulty in displaying GGNs in specific lung locations, but was sensitive to GGNs with CT values of >-455 HU, highly possibly lung invasive adenocarcinomas , giving a hint to treatment strategies. In contrast, T2WI-FS can detect a smaller size of 6 mm for GGNs than ZTE-MRI. Overall, ZTE might play a unique role in differentiating invasive adenocarcinomas from precursor glandular lesions and minimally invasive adenocarcinoma (MIA) in eligible GGNs.Introduction
Screening and detecting lung pathological abnormality at an early stage is critical to increasing survival rate and quality of life for patients1. Ground-glass nodules (GGNs) should be noticed as they have malignancy potential and heterogeneous features, computed tomography (CT) is currently the first-choice approach to detect GGNs2. In the version 1.1 of Lung Imaging Reporting and Data System (Lung-RADS)3, long-term tracking the size of GGNs is essential, but times of radiation exposure to patients may elevate possibility of subsequent cancer progression. A novel ZTE-MRI technique with the feature of nominal zero echo time to capture less-proton tissues with ultrashort transverse relaxation time (T2/T2*) may offer extra information other than CT for lung cancer patients4–7. Diagnostic performance on detecting GGNs using ZTE-MRI has not validated yet; therefore, the present study aimed to evaluate the performance of ZTE-MRI on detection rate of GGNs in comparison with T2WI and liver imaging with volume acceleration-flexible (LAVA-Flex) using CT as reference.Material and Methods
From January to June 2022, 28 patients (male to female ratio=2:5; median age =48 [27–63] years, the presence of GGNs [n=35] confirmed by chest CT scans) underwent chest MRI within 1–4 days after breath-hold CT examination (reconstructed at 1 mm thickness). MR scans were obtained using a 3.0T scanner (750W, GE Healthcare, Milwaukee, USA) including routine axial (Ax) fat-saturated FSE T2WI (T2WI-FS) (TR>3000ms, TE=30–50ms, FOV=36×36cm2, slice thickness=2.0mm), Ax LAVA-Flex (TR=5.4ms, TE=2.1ms, FOV=42×42cm2, slice thickness=5.0mm), and additional Ax ZTE sequence(TR=300–600ms, TE=0, FOV=42×42cm2, slice thickness=1.5mm). Poor MR image quality such as significant motion artifact or respiratory artifact were excluded. The nodule size (the longest diameter) and morphology (pure, part-solid) on CT as standard reference were evaluated by two radiologists with 10 and 8 years of experience in chest imaging. The Statistical Package for SPSS 25.0 (Chicago, IL, USA) and MedCalc18.2.1 (Ostend, Belgium) were used for statistical analyses. The diagnostic performance was evaluated using binary logistic regression analysis and area under the receiver operating characteristic (ROC) curves (AUC). The sensitivity of nodule detection between the two sequences was compared using McNemar’s test. The unadjusted Cohen’s κ statistic was used to determine the inter-sequence and inter-reader agreement.Results
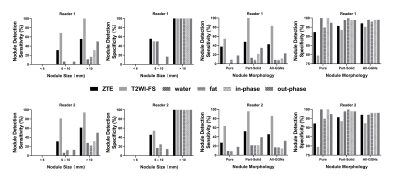
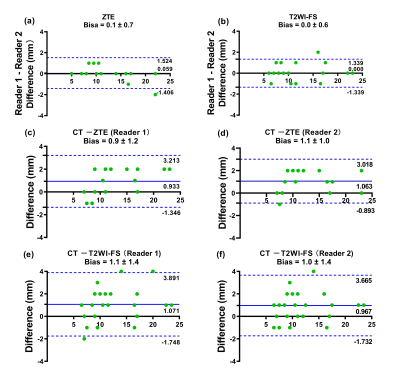
Of the 35 GGNs (average diameter=11.6mm [range 5–24mm]), 11 (31%) were pure and 24 (69%) were part-solid (representative images were shown in Figure 1-2). The sensitivity of nodule detection and diagnostic accuracy for recognizing GGNs were significantly higher on ZTE and T2WI-FS than any other 4 images of LAVA-Flex, including water, fat, in-phase, out-phase (Figure 3). T2WI-FS showed significantly greater sensitivity and diagnostic accuracy of GGNs detection than ZTE according to assessment of both readers (p<0.05). The CT value contributed to significantly elevate GGNs detection rate on ZTE (p<0.05) but not on T2WI-FS (p>0.05), while the gender, age, longest diameter, morphology and pathological type were not. The inter-reader agreement was good in the assessment of GGNs detection for both ZTE (κ=0.94) and T2WI-FS (κ=0.68). The nodule size measurements on ZTE were underestimated by a bias up to 0.9±1.2mm compared to CT and 1.1±1.4mm for T2WI-FS (Figure 4).Discussion and Conclusion
This is the first study to evaluate the utility of ZTE-MRI in detecting GGN on a 3.0 T MRI, and indicated ZTE-MRI had significantly higher sensitivity of GGN detection than LAVA-Flex but lower than T2WI-FS. The nodule detection rate of T2WI-FS in the present study was > 82.9% and higher than the report of the highest detection rate of 62% for pulmonary nodules by 8-mm-thickness T2WI8. This attributed to the thinner-slice (2.0 mm) T2WI-FS in the current study. The reason for ZTE-MRI showing lower GGN detection rate than T2WI-FS may be higher fluid content of GGNs, leading to better contrast of lung parenchyma on T2WI-FS than on ZTE. In our study, T2WI-FS can detect the part-solid nodule no smaller than 6mm, consistent with the minimal size criterion for routine follow-up9,10. Due to being sensitive to motion artifacts from breathing and vascular pulsation, ZTE-MRI had difficulty in displaying GGNs in specific locations, including the subpleural, diaphragmatic, parapericardial, and easily led to missed diagnosis or false-positive detection. In the present study, the CT and MR images showed a satisfactory alignment of nodule size measures. ZTE-MRI and T2WI-FS images revealed high agreement on nodule size. We also found that the CT value was an independent factor of GGNs detection for ZTE-MRI, and the threshold was > -455 HU. This CT value was close to the threshold of invasive adenocarcinoma (IAC, CT value > -472 HU)11. Therefore, ZTE might play a unique role in the differentiating IAC from precursor glandular lesions and minimally invasive adenocarcinoma (MIA) in eligible GGNs. That is, ZTE was sensitive to IAC, give a hint to different treatment strategies. Therefore, some patients can receive MR follow-up scans after they were diagnosed as existence of GGNs, thereby avoiding radiation damage from CT. In conclusion, MRI has a satisfactory performance in detecting GGN, especially T2WI-FS. The ZTE outperformed LAVA-Flex but not T2WI-FS in detecting GGNs and could differentiate IAC from precursor glandular lesions and MIA in eligible GGNs. Overall, ZTE-MRI along with T2WI-FS could not only detect GGNs but also give a hint of disease progression during follow-up to assist decision-making on selection of clinical treatments.Acknowledgements
The authors are deeply grateful to all the volunteers participating in this study. The authors thank the following funding sources: the Foundation of Guangzhou Municipal Science and Technology Bureau (202102010253), Guangdong Demonstration Base for Joint Training of Graduate Students (20201), and Open Project Fund of the Sixth Affiliated Hospital of Guangzhou Medical University (2020-11-370). Jieyang Health and Medical Technology Innovation Project (No.71).References
1. Li Y, Tian X, Gao L, et al. Clinical significance of circulating tumor cells and tumor markers in the diagnosis of lung cancer. Cancer Med. 2019;8(8):3782-3792.
2. Lee HY, Lee KS. Ground-glass opacity nodules: histopathology, imaging evaluation, and clinical implications. J Thorac Imaging. 2011;26(2):106-118.
3. ACoR. Lung-Screening Reporting and Data System (LungRADS) V ersion 1.1. 2019. Available online:https://www.acr.org/-/media/ACR/Files/RADS/Lung-RADS/LungRADSAssessmentCategoriesv1-1.pdf?la=en.
4. Larson PEZ, Han M, Krug R, et al. Ultrashort echo time and zero echo time MRI at 7T. MAGMA. 2016;29(3):359-370.
5. Petousi N, Talbot NP, Pavord I, Robbins PA. Measuring lung function in airways diseases: current and emerging techniques. Thorax. 2019;74(8):797-805.
6. Biederer J, Ohno Y, Hatabu H, et al. Screening for lung cancer: Does MRI have a role? Eur J Radiol. 2017; 86:353-360.
7. Tiddens HAWM, Kuo W, van Straten M, Ciet P. Paediatric lung imaging: the times they are a-changin’. Eur Respir Rev. 2018;27(147):170097.
8. Sommer G, Tremper J, Koenigkam-Santos M, et al. Lung nodule detection in a high-risk population: comparison of magnetic resonance imaging and low-dose computed tomography. Eur J Radiol. 2014;83(3):600-605.
9. Callister MEJ, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70 Suppl 2: ii1-ii54.
10. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for Management of Incidental Pulmonary Nodules Detected on CT Images: From the Fleischner Society 2017. Radiology. 2017;284(1):228-243.
11. Lee HY, Choi YL, Lee KS, et al. Pure ground-glass opacity neoplastic lung nodules: histopathology, imaging, and management. AJR Am J Roentgenol. 2014;202(3): W224-233.
Figures
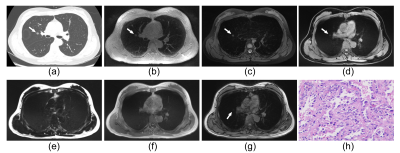
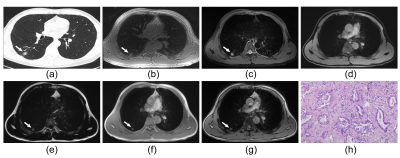
Figure 2: Representative images of a 53-year-old male patient with a part-solid nodule (17mm × 15mm in size) in the right lower lung (a). The nodule can be detected on ZTE (b), T2WI-FS (c) as well as fat (e), in-phase (f) and out-phase (g) of LAVA-Flex. The pathology confirmed invasive adenocarcinoma (HE×100, h).