4818
Feasibility and Reproducibility of Ventilation and Perfusion Imaging at a 0.35 T MR-Linac in Healthy Volunteers and Lung Cancer Patients1Department of Radiology, University Hospital, LMU Munich, Munich, Germany, 2Comprehensive Pneumology Center (CPC-M), Member of the German Center for Lung Research (DZL), Munich, Germany, 3Department of Radiation Oncology, University Hospital, LMU Munich, Munich, Germany, 4Antaros Medical AB, BioVenture Hub, Mölndal, Sweden, 5Univ. Bordeaux, INSERM, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, F-33000 Bordeaux, France, 6German Cancer Consortium (DKTK), Munich, Germany
Synopsis
Keywords: Lung, Cancer, Ventilation, Perfusion
Hybrid MR-imaging and linear accelerators for radiotherapy allow highly accurate treatment of lung cancer patients. The potential of Non-uniform Fourier Decomposition (NuFD), a non-contrast enhanced free-breathing technique that allows the assessment of lung ventilation and perfusion, was investigated in 10 healthy subjects and 2 lung cancer patients at a 0.35 T MR-Linac. Due to challenging ventilation quantification and reproducibility, two normalization strategies were proposed. Evaluation on repeated scans demonstrated clear improvement compared to uncorrected cases. First longitudinal ventilation assessment over treatment course shows promising results for the use of NuFD as treatment response monitoring tool in the clinical workflow.Introduction
Radiation therapy plays an important role in the treatment of lung cancer1. The introduction of combined therapy and MR-imaging devices, called MR-Linacs, in the clinical routine has opened up possibilities in terms of daily adaptive treatment planning and precise tumor localization based on real-time cine-MRI during dose delivery2. Furthermore, these devices potentially allow functional lung imaging in the clinical workflow for treatment response monitoring without prolonging treatments. Several methods have been developed for non-contrast enhanced functional lung MRI at 1.5 T e.g. PREFUL3, SENCEFUL4 or Non-uniform Fourier Decomposition (NuFD)5 to assess ventilation (V) and perfusion (Q). Low-field MRI has been shown to be advantageous for lung imaging due to low susceptibility artefacts6. Therefore, we aim at demonstrating the feasibility and potential of NuFD for a 0.35 T MR-Linac in healthy volunteers and lung cancer patients and assess the performance of two signal normalization strategies for enhancing reproducibility of the results in the presence of breathing amplitude variations.Methods
Ten healthy volunteers (five female, five male, 24-50 y old) were repeatedly scanned with ethics approval at a 0.35 T MR-Linac (MRIdian, ViewRay Inc., Cleveland, Ohio) using an optimized balanced steady-state free precession (bSSFP) sequence with flip angle = 70°, TR/TE = 2.42/1.02 ms, FOV=500×500×20 mm3, matrix = 128×128 and frame rate = 3.68 images/s resulting in a total acquisition time of 1.1 min. Two coronal slices, referred to as ‘aorta’ and ‘lung’ were selected for each volunteer and image series (240 images) were acquired in normal free-breathing with breaks inside and outside the scanner as well as in deep and shallow breathing. Additionally, image series of one coronal slice at the tumor position were obtained for two lung cancer patients (one female, one male, 38 and 80 y old, five and six radiotherapy fractions) in free-breathing right after each radiotherapy treatment session. After image registration to one pre-selected frame using ANTs7 and manual lung segmentation, the average lung signal was filtered into signals corresponding to V and Q using a low- and high-pass filter, respectively. The NuFD was performed pixel-wise and V- and Q-maps were generated from the peak-value in the respective Fourier spectrum. For intra-volunteer/patient V-map reproducibility, a normalization factor was defined based on the linear correlation of V-signal and diaphragm position of each scan as well as the diaphragm motion amplitude of a reference scan. This allows to correct the signal’s dependence on the diaphragm motion amplitude, which varies with the breathing pattern. The second strategy normalizes the V-maps with the average V-signal within a selected ROI and therefore eliminates the dependency on the signal amplitude. In order to study possible performance differences, for all volunteers six different ROI positions in the lung were selected. The two best/worst positions are referred to as ‘best ROI’ and ‘worst ROI’, respectively. For normalization quality assessment, the absolute deviation δ of the mean V-map value between unnormalized/normalized ($$$\bar{V}_{i}$$$) and reference scan ($$$\bar{V}_{ref}$$$) was defined as:$$ \delta = \left| \frac{\bar{V}_{ref}-\bar{V}_{i}}{\bar{V}_{ref}} \right| $$
with i being unnormalized or normalized scans.
Results
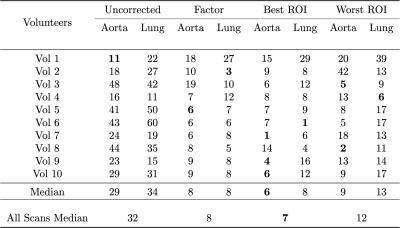
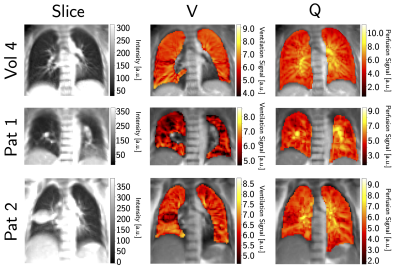
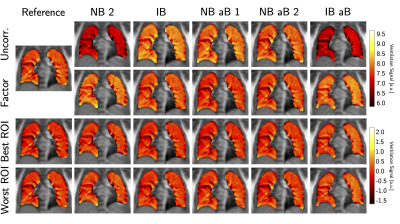
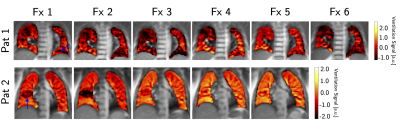
The clear signal variation corresponding to the breathing frequency and the heart beat allowed the successful application of the NuFD algorithm to all volunteer and patient scans and V- and Q-maps were generated (Figure 1). To analyze the normalization results, the V-maps were compared visually (Figure 2) and the median deviations between uncorrected/corrected and reference were determined for both slice positions of all volunteers (Table 1). For the aorta and lung slice deviations of 8%/6%/9% (normalization factor/best ROI/worst ROI) and 8%/8%/13% were achieved. This clearly outperformed the uncorrected deviations of 29% and 34% for the aorta and lung slice, respectively. Combining both slices, a median deviation of 8%/7%/12% was reached compared to 32% for the uncorrected scans. The application of the ROI-based method on the patient data (Figure 3) shows an improving ventilation in the tissue surrounding the tumor for patients 1 and 2 over fraction 1 to 5. The overall V-map intensity decreased for patient 1 in fraction 6.Discussion
The investigation of both normalization strategies in healthy volunteers demonstrated statistical improvement of the V-map reproducibility (Table 2). Even though the ROI-based normalization exhibits a slight position dependency, the deviation from the reference was reduced by a factor of 2.8 or more in comparison to the uncorrected scans. This clearly shows the need for a normalization strategy for the assessment of lung ventilation in longitudinal studies. Encouraging results were also obtained for the first application of the NuFD in lung cancer patients that coincided with diagnosis. Since both patients presented increased signal intensity in the V-maps in the longitudinal comparison of the normalized scans, the potential of this technique for treatment response monitoring was illustrated.Conclusion
We showed that non-contrast enhanced functional lung MRI concepts can be transferred to a 0.35 T MR-Linac. For the comparison of intra-volunteer/patient scans, two signal normalization strategies have been successfully introduced and demonstrated comparable performance. With no need for dedicated equipment, short acquisition and no computationally expensive post-processing, this method is easily integrable into the clinical treatment workflow. Its benefits and potential for treatment monitoring will be further investigated in an ongoing ethics approved patient study.Acknowledgements
No acknowledgement found.References
1. Crockett CB, Samson P, Chuter R, et al. Initial Clinical Experience of MR-Guided Radiotherapy for Non-Small Cell Lung Cancer. Front Oncol. 2021;11:617681.
2. Klüter S. Technical design and concept of a 0.35 T MR-Linac. Clin Transl Radiat Oncol. 2019;18:98-101.
3. Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79(4):2306-2314.
4. Fischer A, Weick S, Ritter CO, et al. SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) using a quasi-random fast low-angle shot (FLASH) sequence and proton MRI. NMR Biomed. 2014;27(8):907-917.
5. Bondesson D, Schneider MJ, Gaass T, et al. Nonuniform Fourier-decomposition MRI for ventilation- and perfusion-weighted imaging of the lung. Magn Reson Med. 2019;82(4):1312-1321.
6. Hori M, Hagiwara A, Goto M, et al. Low-Field Magnetic Resonance Imaging: Its History and Renaissance. Invest Radiol. 2021;56(11):669-679.
7. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54(3):2033-2044.
Figures