4817
Diffusion of 129Xe in the lung: airspace size heterogeneity and airway connectivity assessed by filter exchange imaging1Institute for Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany, 3Clinical Airway Research, Fraunhofer Institute for Toxicology and Experimental Medicine (ITEM), Hannover, Germany, 4Department of Respiratory Medicine, Hannover Medical School, Hannover, Germany
Synopsis
Keywords: Lung, Hyperpolarized MR (Gas)
A filter exchange imaging (FEXI) sequence was developed for studying dynamic changes of apparent diffusion coefficient (ADC) in hyperpolarized 129Xe MRI, thereby probing heterogeneity and connectivity of lung airspaces. The sequence was tested in healthy volunteers and patients of chronic obstructive pulmonary disease (COPD). An initial reduction of ADC after filter application was observed in all subjects. Relative reduction and recovery rate tended to be increased in COPD. Weak/moderate correlations of initial reduction and recovery rate with ADC before filter application were observed. 129Xe FEXI lung MRI is feasible and derived quantities may be useful for disease phenotyping in COPD.Introduction
Chronic obstructive pulmonary disease (COPD) is a category of disease subtypes characterized by airflow obstruction and frequently associated with emphysematous destruction of alveolar structures. Historically slow progress in the development of treatment options may have been caused partly by insufficient phenotyping of disease subtypes which could potentially be done with the help of novel imaging biomarkers.It has been acknowledged in previous studies that diffusion of 129Xe inside an imaging voxel in the lung may be described as a superposition of different diffusion coefficients associated with airspace size heterogeneity.1,2 Multiple b-value acquisition of stretched-exponential signal decay was then used for calculation of average local airspace size. This approach does not allow for assessment of connectivity of compartments with different airspace size, however.
Here, we utilize an alternative concept named filter exchange imaging3,4 (FEXI) to selectively saturate magnetization in fast-diffusing 129Xe spins and sample the time evolution of the 129Xe apparent diffusion coefficient (ADC) to study heterogeneity of airspace sizes and the connectivity of compartments. Purpose of this work was to develop a 129Xe FEXI pulse sequence and to investigate dynamic changes of ADC in a preliminary study with COPD patients and healthy volunteer.
Methods
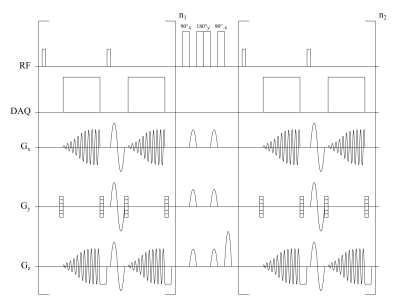
A sequence was implemented according to the sequence diagram in figure 1. Compared to the standard implementation3, the sequence makes use of a low flip angle gradient-echo acquisition and a Look–Locker like acquisition scheme with fast spiral readouts5 to facilitate use with hyperpolarized gases. Sequence parameters are summarized in table 1.This study was approved by the institutional review board and all subjects gave written informed consent. Imaging was performed at 1.5T (Avanto, Siemens) using a 129Xe birdcage transmit coil and 16-channel phased-array receive coil (Rapid Biomedical). 129Xe was hyperpolarized (Model 9810, Polarean) and diluted with N2 to 1L. Subjects inhaled the gas mixture from functional residual capacity and held their breath.
ADC maps were generated and a whole-lung average calculated. A single exponential was assumed for the time dependence of ADC after filter application and the following model function fitted to the data3, $$\mathrm{ADC}(t_m)=\mathrm{ADC}_0(1–a\mathrm{exp}(–Rt_m)),$$ with mixing time tm, initial ADC reduction a and recovery rate R. ADC0 was taken as the average ADC before filter application. The last time point was corrupted due to low SNR in several subjects and thus excluded from analysis in all subjects.
Results
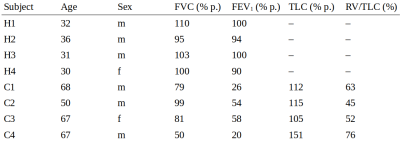
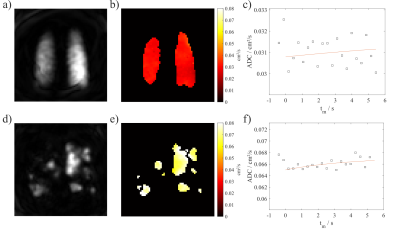
Four healthy volunteers and four COPD patients were included. Table 2 summarizes subject demographics along with results from lung function testing/body plethysmography (only COPD). Figure 2 shows representative 129Xe ADC maps and temporal dynamics of ADC averaged over the whole lung.As expected, ADC0 was increased in COPD patients compared to healthy volunteers, $$$p=0.029$$$. The parameter a was significantly different from zero in the study cohort, $$$p=0.008$$$. Non-significant trends for increased a and R were observed in COPD.
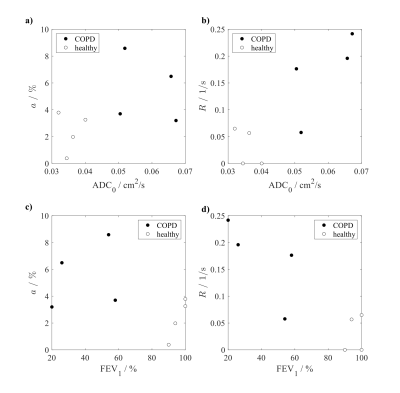
Figure 3 shows scatter plots of the quantities a and R versus ADC0 and FEV1. Weak to moderate correlations of a with ADC0, $$$r=0.333$$$, $$$p=0.428$$$, and of R with ADC0, $$$r=0.643$$$, $$$p=0.096$$$, are observed, respectively. A significant inverse correlation is found in the study cohort between R and FEV1, $$$r=-0.743$$$, $$$p=0.042$$$.
Discussion
In this work the proposed FEXI pulse sequence was found to be feasible in healthy subjects and COPD patients for studying ADC dynamics after selective saturation of fast-diffusing spins, probing heterogeneity and connectivity of lung microstructure. Assuming a free diffusion coefficient D0 of 0.14cm2/s and a RMS displacement of$$$\sqrt{6D_0t}$$$, the spins should be largely confined within an imaging voxel at the beginning of the recovery curve suggesting that the proposed sequence is able to measure airspace size heterogeneity and connectivity on a sub-voxel scale. As suggested by the scatter plot in figure 3a, airspace enlargement reflected by increased ADC0 in COPD may be associated with a variable degree of ADC reduction after filter application, hence sub-voxel-scale tissue heterogeneity. The recovery rate R could be helpful to study the effects of diffusional screening,6 which is thought to be reduced in emphysema, potentially leading to faster recovery.7Being originally proposed as a measurement for intra- and extracellular water exchange,4 we assume the contribution of exchange of 129Xe between tissue and airspaces to be negligible for our FEXI measurements due to the small magnetization of the dissolved phase. The parameter ADC0 in the model function may in fact differ from the averaged ADC before application of the filter due to the non-renewable magnetization of 129Xe and possibly slow recovery in complex media3. The number of time points to be sampled after filter application is limited by SNR degradation at late times and breathhold capabilities. Assuming a fixed ADC0 is expected to stabilize the fits, however, and thus improve repeatability. There may in fact also be a distribution of recovery rates R involved which could potentially be obtained by inverse Laplace transform of the ADC recovery curve.
The influence of choice of filter gradients on results and image SNR remains to be investigated. Future work will concentrate on larger cohorts and the analysis of repeatability of individual parameters.
Conclusion
The proposed sequence is a feasible method for studying sub-voxel-scale lung tissue heterogeneity and may add additional biomarkers for disease phenotyping in COPD.Acknowledgements
This work was funded by the German Center for Lung Research (DZL).References
1. Parra-Robles J, Marshall H, Hartley RA, Brightling CE, Wild JM. Quantification of lung microstructure in asthma using a 3He fractional diffusion approach. In: Proceedings of the 22nd annual meeting of ISMRM, 2014. p. 3529.
2. Ouriadov A, Lessard E, Sheikh K, Parraga G. Pulmonary MRI Morphometry Modeling of Airspace Enlargement in Chronic Obstructive Pulmonary Disease and Alpha-1 Antitrypsin Deficiency. Magn Reson Med. 2018;79:439-448. doi:10.1002/mrm.26642
3. Khateri M, Reisert M, Sierra A, Tohka J, Kiselev VG. What does FEXI measure? NMR Biomed. Published online September 8, 2022. doi:10.1002/nbm.4804
4. Åslund I, Nowacka A, Nilsson M, Topgaard D. Filter-exchange PGSE NMR determination of cell membrane permeability. J Magn Reson. 2009;200(2):291-295. doi:10.1016/j.jmr.2009.07.015
5. Kern AL, Gutberlet M, Moher Alsady T, et al. Investigating short-time diffusion of hyperpolarized 129 Xe in lung air spaces and tissue: A feasibility study in chronic obstructive pulmonary disease patients. Magn Reson Med. 2020;84(4):2133-2146. doi:10.1002/mrm.28264
6. Sapoval B, Filoche M, Weibel ER. Smaller is better—but not too small: A physical scale for the design of the mammalian pulmonary acinus. Proc Natl Acad Sci USA. 2002;99(16):10411–10416. doi:10.1073/pnas.122352499
7. Sapoval B, Filoche M. Role of Diffusion Screening in Pulmonary Diseases. In: Poulin M, Wilson R, eds. Integration in Respiratory Control. Advances in Experimental Medicine and Biology. Springer; 2008:173–178.
Figures