4812
Repeatability and reproducibility of oxygen-enhanced MRI of the lung at 3 tesla: cross-center, cross-vendor evaluation1Centre for Medical Image Computing (CMIC), Department of Medical Physics & Biomedical Engineering, University College London, London, United Kingdom, 2Bioxydyn Limited, Manchester, United Kingdom, 3BHF Manchester Centre for Heart and Lung Magnetic Resonance Research (MCMR), Manchester, United Kingdom, 4Division of Cancer Sciences, University of Manchester, Manchester, United Kingdom, 5Division of Radiotherapy and Imaging, Institute of Cancer Research, Manchester, United Kingdom
Synopsis
Keywords: Lung, Contrast Mechanisms, oxygen-enhanced MRI
A recently developed protocol with a dual echo RF-spoiled gradient echo acquisition enabled simultaneous measurement of dynamic OE signal change and the substantial ∆R2* effect at 3 tesla. To progress towards clinical translation, we evaluated repeatability and reproducibility across two sites, vendors and time points. Our results demonstrate good repeatability for the quantitative and semi-quantitative indices across time points, consistent signal behaviour and repeatable ∆R2* across two sites and vendors, suggesting potential utility in multi-centre clinical studies. However, echo time dependence should be considered when interpreting percentage signal enhancement.Introduction
Oxygen-enhanced MRI (OE-MRI) can provide information on regional lung function [1]. Despite technical challenges of OE-MRI in the lung at higher magnetic field, we have recently shown the feasibility of lung OE-MRI at 3 T and signal enhancement behaviour in the lung using a dual echo RF-spoiled gradient echo acquisition [2]. This method enables measurement of dynamic OE signal change with either T1-weighting or T2*-weighting, and for monitoring of dynamic ∆R2* [2,3]. To translate these biomarkers into clinical use, they must demonstrate their repeatable and reproducible assessment of lung function. The purpose of this work was to evaluate the proposed method across two sites, vendors and time points.Subjects & Methods
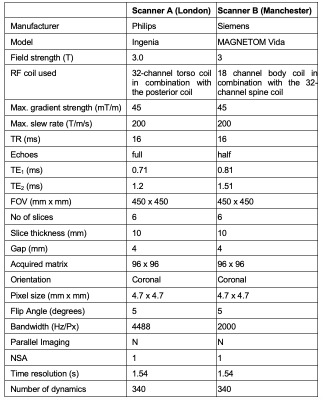
Following research ethics approval and written informed consent, we recruited 16 healthy volunteers with no previous record of lung diseases. Of these, 8 travelling subjects (3 males, age range = 26-54 years, median = 39.5) underwent lung MRI on a 3 T whole-body scanner from two vendors in different cities (Philips Ingenia in London, UK and Siemens MAGNETOM Vida in Manchester, UK) at a 4 week interval. Eight separate subjects (4 males, age range = 23-51 years, median = 27) were scanned twice for a scan-rescan test at a 4-6 week interval using the Philips scanner. Scan parameters for the RF-spoiled dual gradient echo acquisition for each vendor are listed in Table 1. Selection of flip angle (FA) was optimized through simulation (Fig. 1) by varying FA and TE values [2,4]. The shortest TE values available for the chosen acquisition were selected for each scanner: TE1A = 0.71 ms, TE2A = 1.2 ms for Philips; TE1B = 0.81 ms, TE2B = 1.51 ms for Siemens. Subjects breathed medical air (21% O2) and 100% O2 via non-rebreathing face mask (Intersurgical Ltd., Wokingham, UK) at a flow rate of 15 Lmin-1. Images were acquired during free breathing.Data analysis was performed in MATLAB R2022b (MathWorks, Natick, MA). After non-linear image registration using ANTs [5,6], the lung parenchyma, excluding central major vasculature, was manually segmented from registered images and a voxel-wise tissue density correction was applied. Subsequently, the dynamic data points in the masked lung were fitted using exponential functions for the O2 wash-in and wash-out with least-squares algorithm for each voxel. Percent signal enhancement (PSE) maps were produced by the subtraction of the fitted baseline normoxia intensity from the fitted hyperoxia intensity, normalized to the fitted normoxia intensity. Mean and standard deviation (std) were calculated for PSE values at each TE over 6 slices for TE1A, TE1B, TE2A and 2 most posterior slices only for TE2B due to poor signal to noise ratio (SNR) in the anterior slices at TE2B. Mean ∆R2* maps were calculated by the subtraction of averaged normoxia R2* maps (30th to 60th time series acquisitions) from averaged hyperoxia R2* maps (120th to 180th). Intra-class correlation coefficient (ICC) was calculated using SPSS v28.0 (SPSS Inc, Chicago, IL).
Results
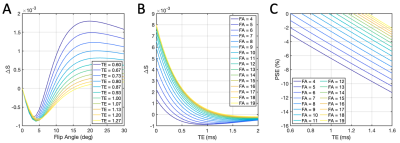
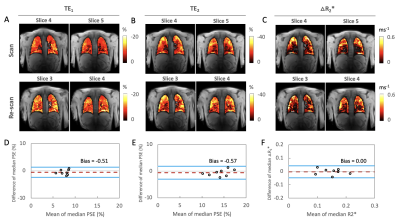
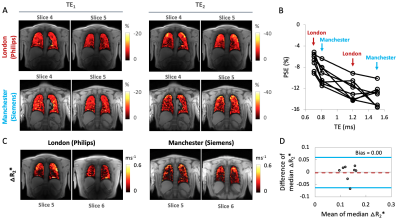
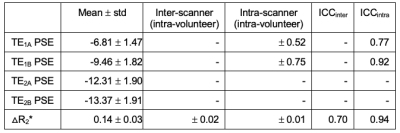
Simulations show that T2*-induced negative enhancement (∆S) is maximum at FA ~5° independent of the choice of TE (Fig. 1A), and TE dependence of ∆S reduces with smaller FA (Fig. 1B). Negative PSE increases with lower FA and longer TE (Fig. 1C).Representative volunteer PSE maps at TE1A (Fig 2A), TE2A (Fig. 2B) and △R2* (Fig. 2C) demonstrate good intra-scanner, intra-subject repeatability, confirmed by Bland-Altman analysis (Fig. 2D-F) (Philips in London). PSE maps from inter-scanner, intra-subject repeat scans show similar spatial distribution of the enhancement signal at TE1 while the signal at TE2 from the Manchester site is compromised due to low SNR caused by longer TE (Fig. 3A). Plots of the combined PSE values at 4 separate TEs from two MRI systems display TE dependence of the signal (Fig. 3B, Table 2) similar to simulation expectations. The inter-scanner variability (± std of the parameter differences between two scanners when the same volunteer was scanned) and the intra-scanner variability (between two sessions when the same volunteer was scanned) show good repeatability and reproducibility of the indices (Table 2), confirmed by △R2* maps and Bland-Altman analysis (Fig. 3C, 3D). High ICC values are observed for both inter-scanner and intra-scanner △R2* comparisons (Table 2).
Discussion
Our results show that the proposed protocol with a dual echo RF-spoiled gradient echo acquisition at 3 T yields excellent intra-scanner repeatability. They also demonstrate that comparable OE-MRI protocols of the lung can be implemented at 3 T across different sites and scanners with good repeatability and reproducibility for △R2*. Although matching TE between scanners is challenging, the expected trend of PSE with TE is observed between sites.Conclusions
We have evaluated repeatability and reproducibility of OE-MRI of the lung at 3 T across two sites, vendors and time points using a dual echo RF-spoiled gradient echo acquisition. Our results demonstrate good repeatability for the quantitative and semi-quantitative indices across time points, consistent signal behaviour and repeatable ∆R2* across two sites and vendors, suggesting potential utility in multi-centre clinical studies. However, substantial impact of TE should be considered when interpretating PSE.Acknowledgements
This work is supported by the Cancer Research UK National Cancer Imaging Translational Accelerator (NCITA) awards C1519/A28682 (UCL) and C19221/A28683 (University of Manchester), the EPSRC-funded UCL Centre for Doctoral Training in Medical Imaging (EP/L016478/1; EP/S021930/1), an EPSRC Industrial CASE award (Voucher No. V20000074), GlaxoSmithKline Research and Development Ltd (BIDS3000035683), and Innovate UK award 104629. We thank Lucy Caselton, Sumandeep Kaur and David M. Higgins for the technical assistance.References
1. Edelman RR et al., Noninvasive assessment of regional ventilation in the human lung using oxygen–enhanced magnetic resonance imaging. Nat Med. 1996; 2: 1236–1239.
2. Kim M et al., Oxygen-enhanced MRI of the lung at 3 tesla: R2* contrast in smokers and non-smokers. Proc Intl Soc Magn Reson Med. 2022; 1481.
3. S. H. Needleman et al., Functional Lung MRI at 3.0 T using Oxygen-Enhanced MRI (OE-MRI) and Independent Component Analysis (ICA), Proc Intl Soc Magn Reson Med. 2022; 658.
4. Kruger SJ et al. Oxygen-enhanced 3D radial ultrashort echo time magnetic resonance imaging in the healthy human lung. NMR Biomed. 2014; Dec;27(12):1535-41.
5. Avants BB et al. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med Image Anal. 2008;12(1):26-41.
6. Avants BB et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033-2044.
7. Dietrich et al. Oxygen-enhanced MRI of the lung at 3 Tesla: Feasibility and T1 relaxation times. Proc Intl Soc Magn Reson Med. 2006; 14: 1307.
Figures