4804
Single-shot 2D Radial Echo Planar Imaging using a KWIC Filter and Model-based Reconstruction Approach1Medicine, University of Hawaii, Honolulu, HI, United States, 2Univeristy of Hawaii, Honolulu, HI, United States, 3University of Hawaii, Honolulu, HI, United States
Synopsis
Keywords: Data Acquisition, Brain
High quality brain images at spatial resolutions of 2x2x3 mm3 and 3.3x3.3x3 mm3 were obtained using single-shot 2D rEPI. The approach is based on an R2*, B0 and coil sensitivity informed model-based reconstruction in combination with k-space weighted image contrast (KWIC) filtering. Signal loss due to phase inconsistencies are starkly reduced by the linear phase model and R2* contrast was modifiable by adjusting the target echo time in the model of the reconstruction and KWIC filter. Bold activation maps were generated from fMRI datasets at 2x2 mm2 resolution showing activation in the visual cortex.INTRODUCTION
Echo planar imaging (EPI) is one of the most significant fast MRI acquisition methods and is readily used in dynamic imaging applications such as functional MRI (fMRI) and perfusion imaging (1-8) as well as diffusion tensor imaging. More recently, variations of EPI type sampling have been applied in simultaneous multi-parametric (SMP) mapping (9-13). Despite the large impact of EPI, the development of a radial analogue has proven to be challenging and only in recently acquisitions using radial EPI (rEPI) type sampling have received growing attention again.(14-18) Potential advantages of rEPI include its ability to generate distortion-free images, its incoherent undersampling characteristics and the continuous update of the k space center during the readout.(19) However, a major difficulty also is associated with the latter in the combination of multiple echo lines during rEPI image reconstruction. Left unaccounted for, the signal evolution between different echo lines leads to stark inconsistencies in the k space data at low spatial frequency and therefore poor results in the final images. Most current implementations use multiple shots and/or short echo time span/combine only a few echo lines to mitigate the effect.(20-23) The approach described here uses an R2*, B0 and coil sensitivity informed model-based reconstruction in combination with k-space weighted image contrast (KWIC) filtering(24) and was shown to enable single-shot rEPI of the brain at 2x2 mm2 in plane resolution.METHOD
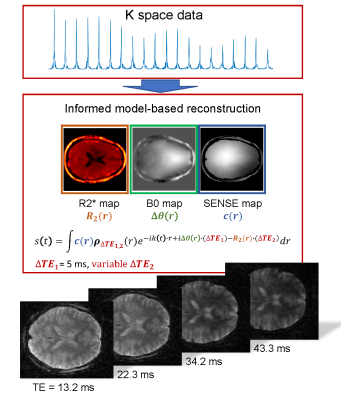
Trajectory Design: A single-shot rEPI trajectory consists of a sequence of radial readout gradients rotated around the k space center by gradient blips such that the entire 2D plane is sampled in a single-shot. In our implementation a small golden angle rotation (φ=27.198…°) was used between consecutive echo lines in the rEPI echo train (Fig. 1).(25) The angle of the rotation presents a well-suited compromise between distributing consecutive echo lines over the rotation span to introduce temporal incoherency and minimizing down time between echo lines for high sampling efficiency. Simultaneously, the elements of the generalized Fibonacci sequence (20, 33, 53) of the small golden angle rotation present effective transition points for the implementation of the KWIC filter (Fig. 1).(26) The radii of the latter were chosen to sample the inner section of k space up to 65% of the full radius at an undersampling factor of less than two (R≤2) of the Nyquist sampling criterium.Acquisition: Brain MRI data for 2x2 mm2 and 3.3x3.3 mm2 in plane resolution were acquired on a Siemens 3T Prisma MRI using a 2D interleaved multislice spoiled radial EPI sequence (Nslice=24, wslice=3mm, TR=1690/1510ms, and nTE=53, TE(1)=5.0/4.9 ms, ΔTE=0.9/0.8 ms) covering a 72 mm-thick region of the brain (FOVxy=210mm). Flip angles of 40° and 70° were used for the T2w brain images (Fig. 2) and fMRI time series (Fig. 3), respectively. The fMRI tasks consisted of 6 blocks of 20 s flickering checkerboard for visual stimulation separated by 20-second resting periods leading to a total duration of the paradigm of 4:20 min.
Reconstruction: Retrospective KWIC filtering was applied to the radial EPI data as described above. An iterative reconstruction modeling coil sensitivities (SENSE),(27) B0 field inhomogeneities, and transversal relaxation within the signal equation was used to generate final images. B0 Field maps (Projection onto Dipole Fields)(28) R2* maps (mono-exponential fitting), and coil sensitivity maps (adaptive array-combination technique)(29) were obtained from a separate radial multi-echo acquisition. Non-uniform fast Fourier transform (NUFFT) on k space data was performed using (NUFFT-toolbox)(30) and combined with a limited-memory BFGS (Poblano toolbox) algorithm for minimization. All reconstructions were performed offline using Matlab®. BOLD analyses were carried out using a general linear model with a canonical hemodynamic response function after removal of first and second order temporal trends in the data. No spatial smoothing, masking, or corrections for multiple comparisons was applied.
RESULTS
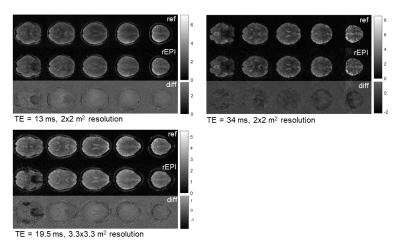
High quality brain images at spatial resolutions of 2x2x3 mm3 and 3.3x3.3x3 mm3 were obtained using the single-shot 2D rEPI approach (Fig. 2). The TA for a 24-slice acquisition was 1.69 ms and 1.51 ms, respectively. Signal loss due to phase inconsistencies are starkly reduced by the linear phase model (Fig. 2) and R2* contrast was modifiable by adjusting the target echo time in the model of the reconstruction and KWIC filter (Fig 2). Bold activation maps were generated from fMRI datasets at 2x2 mm2 resolution. Activation in the visual cortex was clearly visible (Fig 3).DISCUSSION
The importance of modelling the signal evolution over the echo time space to obtain high quality single-shot rEPI images is clearly reflected in our results. However, there is potential for bias being introduced by the model and additional testing is needed. Currently, our approach is based on quality maps from an extra scan prior to the actual single-shot acquisition to inform the reconstruction. Alternative techniques to derive them are strongly warranted to avoid potential misalignment issues and other inconsistencies between maps and acquisition as well as to increase significance of the rEPI method for other applications.CONCLUSION
To our knowledge this is the first time single-shot rEPI has been successfully applied to obtain brain images with high image quality. Further development of this technique, however, is still needed to improve the significance of rEPI for other imaging modalities.Acknowledgements
This project was supported by the NIH grants 1P20GM139753-01A1) and R01 EB023618.References
1. Mansfield P. Multi-planar image formation using NMR spin echoes. J Phys C 1977;10:L55-L58.
2. Bandettini PA, Wong EC, Hinks RS, Tikofsky RS, Hyde JS. Time course EPI of human brain function during task activation. Magn Reson Med 1992;25:390-397.
3. Kwong KK, Belliveau JW, Chesler DA, Goldberg IE, Weisskoff RM, Poncelet BP, Kennedy DN, Hoppel BE, Cohen MS, Turner R, Cheng H-M, Brady TJ, Rosen BR. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc Natl Acad Sci, USA 1992;89:5675-5679.
4. Ogawa S, Tank DW, Menon R, Ellerman JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: Functional brain mapping with magnetic resonance imaging. Proc Natl Acad Sci USA 1992;89:5951-5955.
5. Turner R, Jezzrad P, Wen H, Kwong KK, Le Bihan D, Zeffiro T, Balaban RS. Functional Mapping of the Human Visual Cortex at 4 and 1.5 Tesla Using Deoxygenation Contrast EPI. Magnetic Resonance in Medicine 1993;29:277-279.
6. Warach S, Wielopolsk P, Edelman R. Identification and characterization of the ishemic penumbra of acute human stroke using echo planar diffusion and perfusion imaging. Proc Soc Magn Reson Med; 1993; New York, NY. p 249. (Proc Soc Magn Reson Med).
7. Edelman R, Siewert B, Darby D, Thangaraj V, Nobre A, Mesulam M, Warach S. Qualitative Mapping of Cerebral Blood Flow and Functional Localization with Echo-planar MR Imaging and Signal Targeting with Alternating Radio Frequency. Radiology 1994;192:513-520.
8. Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophysical J 1994;66:259-267.
9. Ordidge RJ, Gibbs P, Chapman B, Stehling MK, Mansfield P. High-speed multislice T1 mapping using inversion-recovery echo-planar imaging. Magn Reson Med 1990;16(2):238-245.
10. Clare S, Jezzard P. Rapid T(1) mapping using multislice echo planar imaging. Magn Reson Med 2001;45(4):630-634.
11. Renvall V, Witzel T, Wald LL, Polimeni JR. Automatic cortical surface reconstruction of high-resolution T1 echo planar imaging data. Neuroimage 2016;134:338-354.
12. Cohen O, Polimeni JR. Optimized inversion-time schedules for quantitative T1 measurements based on high-resolution multi-inversion EPI. Magn Reson Med 2018;79(4):2101-2112.
13. Wang F, Dong Z, Reese TG, Bilgic B, Katherine Manhard M, Chen J, Polimeni JR, Wald LL, Setsompop K. Echo planar time-resolved imaging (EPTI). Magn Reson Med 2019;81(6):3599-3615. 14. Silva AC, Barbier EL, Lowe IJ, Koretsky AP. Radial echo-planar imaging. J Magn Reson 1998;135(1):242-247.
15. Saucedo A, Macey PM, Thomas MA. Accelerated radial echo-planar spectroscopic imaging using golden angle view-ordering and compressed-sensing reconstruction with total variation regularization. Magn Reson Med 2021;86(1):46-61.
16. Schneider M, Benkert T, Solomon E, Nickel D, Fenchel M, Kiefer B, Maier A, Chandarana H, Block KT. Free-breathing fat and R2* quantification in the liver using a stack-of-stars multi-echo acquisition with respiratory-resolved model-based reconstruction. Magnetic Resonance in Medicine 2020;84(5):2592-2605.
17. Benkert T, Feng L, Sodickson DK, Chandarana H, Block KT. Free-breathing volumetric fat/water separation by combining radial sampling, compressed sensing, and parallel imaging. Magnetic Resonance in Medicine 2017;78(2):565-576.
18. Lee GR, Griswold MA, Tkach JA. Rapid 3D radial multi-echo functional magnetic resonance imaging. NeuroImage 2010;52(4):1428-1443.
19. Graedel NN, McNab JA, Chiew M, Miller KL. Motion correction for functional MRI with three-dimensional hybrid radial-Cartesian EPI. Magn Reson Med 2017;78(2):527-540.
20. Wang X, Rosenzweig S, Scholand N, Holme HCM, Uecker M. Model-based reconstruction for simultaneous multi-slice mapping using single-shot inversion-recovery radial FLASH. Magnetic Resonance in Medicine 2021;85(3):1258-1271.
21. Christoph Rettenmeier, Danilo Maziero, K. Tobias Block, Stenger VA. Simultaneous Multi-Slice Radial Echo Volumar Imaging for Fast Simultaneous Multi-Parametric Imaging. Proc Intl Soc Mag Reson Med 27 (2020) 2020;Abstract 0622.
22. Christoph Rettenmeier, Danilo Maziero, Stenger VA. Twisted radial echo planar trajectory (EPIstar) for 3D self-navigated golden angle structural and functional MRI. Proc Intl Soc Mag Reson Med 27 (2019) 2019;Abstract 4581.
23. Zhengguo Tan, Uecker M. Radial Echo-Planar Imaging with Subspace Reconstruction for Brain MRI. Proc Intl Soc Mag Reson Med 30 (2022) 2022;Abstract 1860.
24. Neumann D, Breuer FA, Völker M, Brandt T, Griswold MA, Jakob PM, Blaimer M. Reducing contrast contamination in radial turbo-spin-echo acquisitions by combining a narrow-band KWIC filter with parallel imaging. Magnetic Resonance in Medicine 2014;72(6):1680-1686.
25. Wundrak S, Paul J, Ulrici J, Hell E, Geibel M-A, Bernhardt P, Rottbauer W, Rasche V. Golden ratio sparse MRI using tiny golden angles. Magnetic Resonance in Medicine 2016;75(6):2372-2378.
26. Song HK, Dougherty L. k-space weighted image contrast (KWIC) for contrast manipulation in projection reconstruction MRI. Magn Reson Med 2000;44(6):825-832.
27. Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magnetic Resonance in Medicine 1999;42(5):952-962.
28. Funai AK, Fessler JA, Yeo DTB, Olafsson VT, Noll DC. Regularized Field Map Estimation in MRI. IEEE transactions on medical imaging 2008;27(10):1484-1494.
Figures