4783
Clinical feasibility of artificial intelligence-assisted compressed sensing for accelerated MR imaging in nasopharyngeal carcinoma1Department of Radiology, Sun Yat-sen University Cancer Center, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Guangdong Key Laboratory of Nasopharyngeal Carcinoma Diagnosis and Therapy, Guangzhou, China, 2Shenzhen United Imaging Research Institute of Innovative Medical Equipment, Shenzhen, China, 3MR Collaboration, Central Research Institute, United Imaging Healthcare, Shanghai, China
Synopsis
Keywords: Data Acquisition, Machine Learning/Artificial Intelligence
To improve the scanning efficiency of magnetic resonance imaging (MRI) for nasopharyngeal carcinoma, this study investigated the value of accelerating technique, artificial intelligence-assisted compressed sensing (ACS), in comparison to conventional sequences without accelerating technique and accelerating MRI using parallel imaging (PI). Eleven patients diagnosed with nasopharyngeal carcinoma were prospectively enrolled. As a result, ACS achieved the shortest acquisition time, with similar or even better image quality and SNR than conventional sequences. ACS has the potential to provide sufficient image quality for T1- and T2-weighted imaging in nasopharyngeal carcinoma and could be an alternative to conventional sequences in clinical practice.
Introduction
For nasopharyngeal carcinoma (NPC), a characteristic malignant tumor in Southeast Asia, magnetic resonance imaging (MRI) is the mainstream imaging modality [1]. T1-weighted imaging (T1WI) and T2-weighted imaging (T2WI) remain essential protocols for lesion detection, tumor staging, and treatment response evaluation. Conventional MRI sequences without accelerating techniques or accelerating with parallel imaging (PI) are routinely used in the clinic, which is limited by relatively long acquisition time to some extent. Thus, there is an urgent need to optimize acquisition time as well as maintain high image quality for the imaging of nasopharyngeal carcinoma. Artificial intelligence-assisted compressed sensing (ACS), a work-in-progress technique combining compressed sensing (CS) and convolutional neural network (CNN), is recently been innovatively applied in liver imaging and is considered valuable in performing fast acquisition and remaining the stability of image quality [2, 3]. However, the value of ACS in nasopharyngeal carcinoma imaging is unknown. The goal of this study is to identify the clinical feasibility of ACS-based T1WI and T2WI MRI in shortening acquisition time and evaluate the corresponding image quality, compared with conventional sequences without accelerating technique and with PI in nasopharyngeal carcinoma.Methods
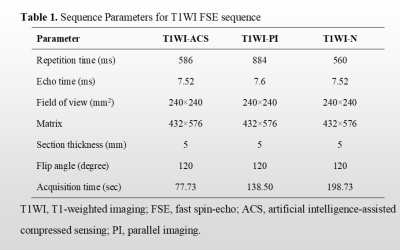
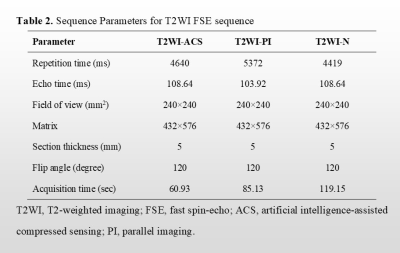
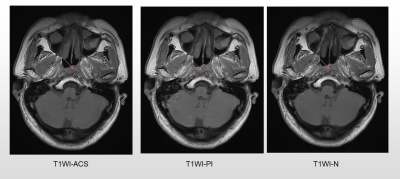
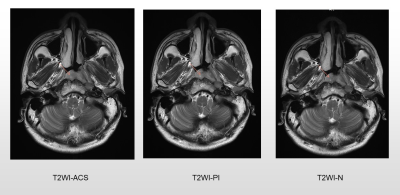
A total of 11 patients newly diagnosed with nasopharyngeal carcinoma were prospectively enrolled. All patients underwent axial T1WI and T2WI fast spin-echo sequences with PI, ACS, and without accelerating techniques (N), respectively at a 3.0 T MR scanner (uMR Omega, United Imaging Healthcare, Shanghai, China). As previously described, for the ACS technique, an extended fully CNN with paired images was utilized in this study to realize the deep learning-based reconstruction [3]. Detailed parameters of each acquisition sequence are shown in Table 1. All image analyses were completed by two experienced radiologists independently. Studies were read in random patient order for each viewing session. The observers were unaware of patients’ information including history, and laboratory results. For qualitative analysis, each reader evaluated the overall image quality on a 5-point scale (1 = poor image quality with no diagnostic value; 2 = substantial deficits, indistinguishable target tissues; 3 = moderate deficits, unaffected target tissues; 4 = minor deficits, slightly obscure target tissues; and 5 = no deficits, well-delineated anatomical structures of target tissues). Besides, conspicuity and distortion of the primary tumors, as well as tumor edge sharpness were also evaluated on the 5-point Likert scale with higher scores representing better image quality. For quantitative analysis, two experienced radiologists independently drew the following regions of interest (ROIs): 1) on the primary tumors, avoiding the cystic and necrotic portions, 2) on the right masseter muscle, 3) on the brain stem, and 4) on the nasopharyngeal air. The signal-to-noise ratio (SNR) of tumors, and contrast-to-noise ratios (CNRs) for the tumor and muscle, as well as tumor and brainstem, were calculated. The Friedman tests were performed to evaluate whether the SNRs and CNRs displayed significant differences among different imaging techniques. Mann-Whitney U test was used to assess differences in ACS vs. PI, ACS vs. N, and PI vs. N. P < 0.05 was considered significant.Results
ACS achieved significantly better image quality for nasopharyngeal tumors, with the shortest scanning time for axial T1WI (77.73s vs. 138.50s vs. 198.73s, P < 0.001) and T2WI (60.93s vs. 85.13s vs. 119.15s, P < 0.001) compared with conventional sequence N and PI. For T1WI sequences, The SNR of tumors in ACS images was significantly higher than those in PI images (P = 0.046), while the difference was not significant when compared with sequence without accelerating techniques. The CNR showed no significant difference among the three sequences. For T2WI sequences, the SNR of tumors in ACS images was significantly higher than those in images of PI (P = 0.025) and N ( P = 0.036). The CNR of tumor and muscle in ACS images was significantly higher than those in images of N ( P = 0.025), while not significant when compared with images of PI.Discussion
Given the AJCC/UICC guideline, patients with NPC are recommended to treat with chemoradiotherapy rather than surgery, thus MRI is critical for tumor assessment [4]. However, conventional T1WI and T2WI with or without accelerating techniques, like half Fourier, PI, and CS, are relatively time-consuming [5,6]. Thus ACS technique combining CS with CNN has been developed, allowing for the advantage in disease detection, structural preservation, and noise suppression [7, 8]. This study innovatively identified the clinical feasibility of ACS-based T1WI and T2WI in nasopharyngeal carcinoma, with significantly reduced acquisition time and remained or improved image quality, SNR, and CNR in comparison to conventional non-accelerating and PI sequences. A larger sample size, coronal and sagittal MRI sequences will be realized in the future to validate the results.Conclusion
T1WI and T2WI images with the ACS technique yielded an optimal performance as they achieved comparable or even better image quality, SNR and CNR with the shortest acquisition time, and could be an alternative to conventional MRI sequences for nasopharyngeal carcinoma.Acknowledgements
No acknowledgement found.References
[1] Chen YP, Chan ATC, Le QT, et al. Nasopharyngeal carcinoma. Lancet 2019;394(10192):64–80.
[2] Li H, Hu C, Yang Y, et al. Single-breath-hold T2WI MRI with artificial intelligence-assisted technique in liver imaging: As compared with conventional respiratory-triggered T2WI. Magn. Reson. Imaging 2022;93:175–180.
[3] Sheng RF, Zheng LY, Jin KP, et al. Single-breath-hold T2WI liver MRI with deep learning-based reconstruction: a clinical feasibility study in comparison to conventional multi-breath-hold T2WI liver MRI. Magn. Reson. Imaging 2021;81:75–81.
[4] Pan JJ, Ng WT, Zong JF, et al. Proposal for the 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 2016;122(4):546–558.
[5] Herrmann J, Gassenmaier S, Nickel D, et al. Diagnostic confidence and feasibility of a deep learning accelerated HASTE sequence of the abdomen in a single breath-hold. Investig. Radiol 2021;56(5):313–9.
[6] Siewert B, Muller MF, Foley M, et al. Fast MR imaging of the liver: quantitative comparison of techniques. Radiology 1994;193(1):37–42.
[7] Schlemper J, Caballero J, Hajnal JV, et al. A deep cascade of convolutional neural networks for dynamic MR image reconstruction. IEEE Trans. Med. Imaging 2018;37(2):491–503.
[8] ] Hu C, Yi Z, Kalra MK,, et al. Low-dose CT with a residual encoder-decoder convolutional neural network (RED-CNN). J. IEEE Trans. Med. Imag 2017;36(99):2524–35.