4782
Improved Clinical Diffusion Weighted Imaging by Combining Deep Learning Reconstruction, Partial Fourier, and Super Resolution1MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany, 2Digital Technology & Innovation, Siemens Medical Solutions USA, Princeton, NJ, United States, 3Pattern Recognition Lab, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 4Institute of Radiology, Martha-Maria Hospital, Nuremberg, Germany
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Translational Studies
Diffusion weighted imaging (DWI) has found widespread use in daily clinical routine but can still be limited by long acquisition times and low spatial resolution. In this work, combining deep learning-based k-space to image reconstruction with super resolution processing tailored to support partial Fourier acquisitions is demonstrated to efficiently mitigate these obstacles. The approach is shown for various applications, including liver, breast, prostate, and brain DWI at 0.55T, 1.5T, and 3T.Purpose
Diffusion weighted imaging (DWI) based on single-shot echo-planar imaging (SS-EPI) has become an indispensable asset in daily clinical routine, where it is being used for various applications spanning the entire body1-3. However, one major limitation is the inherently low signal-to-noise ratio, which is overcome by acquiring multiple repetitions at the cost of long scan times. Furthermore, comparatively low image resolutions are usually chosen to limit the echo train length and the corresponding echo time, which would otherwise lead to further signal reduction due to T2*-decay. Here, clinically established countermeasures are the use of parallel imaging and partial Fourier (PF), which, however, can lead to noise enhancement and image blurring, respectively. To overcome these limitations, we describe a holistic approach which consists of two separate components: (i) deep learning (DL)-based k-space to image reconstruction to enable efficient denoising and (ii) DL-based super resolution with dedicated partial Fourier support to increase image sharpness.Methods
DL-based k-space to image reconstructionThe first step of the proposed processing pipeline is reconstruction of undersampled single-shot k-space data, following the concept of a variational network4. After 6 unrolled iterations with no additional regularization, 11 iterations are performed with a neural network using a hierarchical down-up architecture as regularizer and trainable gradient steps with Nesterov extrapolations. Besides the raw k-space data, precalculated coil sensitivity maps are used as input. The reconstruction was trained in a supervised setting, using about 1,000,000 single repetitions of ground truth images which have been acquired in volunteer scans across different body regions and various clinical 1.5T and 3T scanners (MAGNETOM scanners, Siemens Healthcare, Erlangen, Germany).
DL-based super resolution with dedicated partial Fourier support
To avoid Gibbs-Ringing due to limited k-space sampling, k-space filters are routinely employed which leads to image blurring. This is aggravated if partial Fourier is used to reduce the echo time, since the asymmetric acquisition results in additional blurring along the phase-encoding direction when no dedicated processing is performed. Therefore, the second step of the proposed processing pipeline is to restore image sharpness by removing all k-space filters and employing a DL-based super resolution algorithm instead. For this purpose, a pixel-shuffle architecture5 was trained on ~7000 images, acquired without partial Fourier using DWI and turbo spin-echo sequences in volunteers. By cropping these ground truth images in the frequency domain by a factor of 0.5, corresponding input images were created. Partial Fourier was additionally simulated by zerofilling corresponding frequency parts. Separate networks were trained for different PF factors (PF 5/8, 6/8, 7/8, and 8/8).
Reconstruction pipeline
Training these two independent components was performed offline in PyTorch and afterwards integrated as research application in the reconstruction pipeline of the scanner to allow in-line calculation of images. Image calculation with both DL-based methods is followed by conventional DWI processing such as averaging, trace-weighting, and ADC calculation. To enable optimal load balancing and clinically acceptable reconstruction times, the DL-based k-space to image reconstruction is performed on the scanner’s GPU, while the DL-based super resolution is done on the CPU.
Imaging experiments
The combined reconstruction approach is demonstrated in in-vivo scans at field strengths of 0.55T, 1.5T, and 3T. Applicability is shown for various body regions in volunteers and one patient, including SS-EPI-based DWI of brain, breast, and liver as well as reduced FOV DWI of the prostate using different clinical MR scanners (MAGNETOM scanners, Siemens Healthcare, Erlangen, Germany). Imaging parameters are shown in Table 1. Each dataset was retrospectively reconstructed as follows:
- GRAPPA (“Conventional”)
- DL-based k-space to image reconstruction (“DL-recon”)
- DL-recon and DL-based super resolution (“DL-recon + super resolution”)
Results
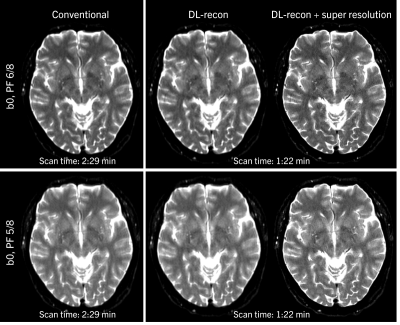
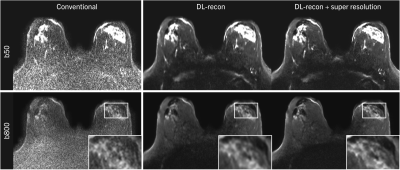
Figure 1 shows the use of DL-recon to reduce acquisition time for brain DWI at 3T while maintaining signal-to-noise ratio (SNR). Additionally applying super resolution increases image sharpness. This is particularly visible in the acquisition with PF 5/8, which otherwise suffers from strong blurring especially along the phase-encoding direction.Results of the high-resolution breast DWI scan are shown in Figure 2. Here, conventionally reconstructed images are non-diagnostic, while the DL reconstruction achieves reasonable SNR.
DWI at low-field often suffers from low SNR, which is shown for a liver scan in Figure 3. By using the proposed combined reconstruction approach, both SNR and sharpness are increased.
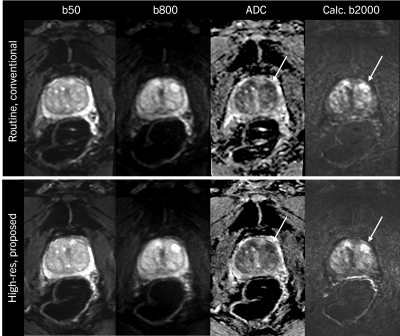
Figure 4 shows results of a patient with suspected prostate cancer. In the clinical routine protocol, spatial resolution is limited, leading to blurred appearance of the lesion. In the high-resolution setting, which is enabled by the proposed reconstruction pipeline, the delineation of the lesion is improved, potentially leading to increased diagnostic confidence.
Discussion and Conclusion
This work demonstrates how the combination of DL-based k-space to image reconstruction and DL-based super resolution processing tailored to support partial Fourier acquisitions enables clinical DWI with increased spatial resolution and reduced scan times. The proposed approach works reliably across different field strengths and different body areas, therefore promising increased efficiency and potentially improved diagnosis in various clinical settings.Disclaimer
The concepts and information presented in this paper are based on research results that are not commercially available. Future commercial availability cannot be guaranteed.Acknowledgements
No acknowledgement found.References
1. Taouli B et al. Diffusion-weighted MR imaging of the liver. Radiology 2010;254:47-66
2. Turkbey B et al. Prostate imaging reporting and data system version 2.1: 2019 update of prostate imaging reporting and data system version 2. Eur Urol 2019;76:340-351
3. Maier SE et al. Diffusion imaging of brain tumors. NMR Biomed 2010;23:849-864
4. Hammernik K et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med 2018;79:3055-71 5. Shi W et al, Real-Time Single Image and Video Super-Resolution Using an Efficient Sub-Pixel Convolutional Neural Network, CVPR 2016;1874-1883
5. Shi W et al, Real-Time Single Image and Video Super-Resolution Using an Efficient Sub-Pixel Convolutional Neural Network, CVPR 2016;1874-1883
Figures