4770
Brain functional network hierarchy and neurochemical correlates in deficit and non-deficit schizophrenia1Huaxi MR Research Center (HMRRC), Functional and molecular imaging Key Laboratory of Sichuan Province, Department of Radiology, West China Hospital, Sichuan University, Chengdu, China., Chengdu, China, 2College of Electronic Engineering, Chengdu University of Information Technology, Chengdu, P.R. China., Chengdu, China
Synopsis
Keywords: Psychiatric Disorders, Gradients
By combining the brain hierarchy changes and their likely potential molecular signatures, we found that the compression of the cortical hierarchy organization in deficit schizophrenia (NS) and non-deficit schizophrenia (NDS) with neurochemical correlates. The altered functional gradients were spatially positively correlated with the receptor of 5-HT2a in DS patients, while negatively correlated with receptor of D1/2 in NDS patients, which revealed the underlying physiological mechanisms of the heterogeneity in schizophrenia.Introduction
Schizophrenia is a heterogenous psychiatric disease, and deficit schizophrenia (DS) is a clinical subgroup with primary and enduring negative symptoms and impaired social function and emotional processing 1. The dopamine hypothesis is the long-standing pathoetiologic theory of schizophrenia 2, which is well explained the positive symptoms in schizophrenia. However, the underlying mechanism of the negative symptoms in DS remain poorly understood. Previous neuroimaging studies have identified functional connectome alterations in schizophrenia, characterizing the extensive brain network functional architecture in DS and non-deficit schizophrenia (NDS) may elucidate their potential biological difference. A novel gradient-based approach 3 has been introduced to define a non-linear decomposition of high-dimensional resting-state functional connectivity (FC) and focused on connectomes where voxels with similar connectivity patterns are located close to one another along a given connectivity gradient. Additionally, to investigate the molecular underpinnings of the brain functional hierarchy, we correlated the functional gradient alterations in patients with DS and NDS and its potential neurochemical architecture.Methods
A total of 154 participants were recruited in this study, including 44 DS, 50 NDS and 60 HC. The magnetic resonance imaging (MRI) scanning of participants was conducted on a GE Signa EXCITE 3.0T scanner (GE Healthcare, Milwaukee, Wisconsin) with an 8-channel phase array head coil. Resting-state functional MRI (rs-fMRI) data and high-resolution T1-weighted images (T1WI) were obtained for all participants. Functional data were preprocessed included the following steps: removal of first five dummy volumes, slice time correction, realignment, segmentation, normalization to the Montreal Neurologic Institute (MNI) space, bandpass filter (0.01-0.10 Hz) and spatial smoothing (full width at half maximum, FWHM = 4 mm). After data preprocessing, the individual brain FC matrix was constructed using Pearson’s correlation between the time courses of each voxel. Gradient metrics were calculated using BrainSpace Toolbox (http://github.com/MICA-MNI/BrainSpace) 3. Voxel-based gradient values were generated and group-averaged gradient values were further extracted across all voxels (global) and 8 networks (including subcortical regions). We assessed the network hierarchy changes by comparing the gradient values at global, network and regional level across the DS, NDS and HC groups. We also examined the relationships between network-level gradient values and clinical features (Positive and Negative Syndrome Scale, PANSS) in DS and NDS patients. Furthermore, we evaluated the spatial correlation between voxel-wise unthresholded T statistic maps of t-test (DS/NDS-induced gradient changes) and brain-wide spatial expression of receptors provided by JuSpace toolbox (https://github.com/juryxy/JuSpace) 4, to investigate the molecular basis underlying changes of functional gradient introduced by DS and NDS patients. We focused on the serotonin receptors (5-HT1a/b and 5-HT2a) and dopamine receptors (D1/2) which were provide by the toolbox derived from prior PET/SPECT studies of healthy volunteers.Results
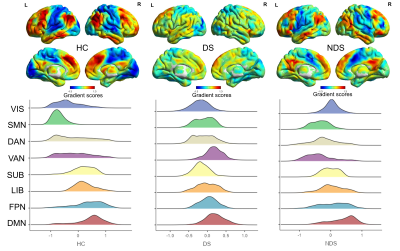
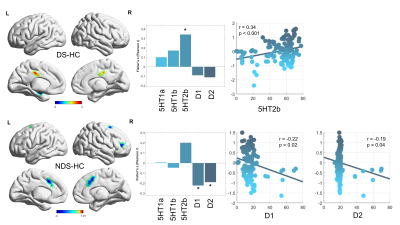
The spatial patterns of the group-averaged principal gradient maps were all along a gradual axis from the primary-to-transmodal gradient in three groups with compression of the cortical hierarchy organization in both DS and NDS group comparing to HC, with a prominent disorganized patterns in DS (Figure 1). Specifically, DS patients showed increased visual network (VIS), sensorimotor network (SMN), dorsal attention network (DAN) and ventral attention network (VAN) and decreased subcortical regions (SUB), limbic network (LIB), frontoparietal network (FPN), default mode network (DMN), while NDS patients demonstrated increased VIS and SMN and decreased DAN, VAN, SUB, LIB, FPN and DMN compared to HC, resulting in a diminished separation in these networks. The increased VAN was positively correlated with the depression scale of PANSS scores in DS patients, while there was no association between aberrant network gradient values and clinical variables in NDS patients. In regional level, the increased left middle cingulate involved in VAN and decreased left hippocampus involved in LIB in DS patients, while multiple decreased medial frontal gyrus involved in the DAN, VAN, LIB in NDS patients. Interestingly, neurochemical correlates showed that altered functional gradients were spatially positively correlated with the 5-HT2a in DS patients, while negatively correlated with D1/2 in NDS patients (Figure 2).Discussion
Combining brain functional hierarchy and its likely potential molecular signatures, we investigated that compression of the cortical hierarchy organization of DS and NDS, and the altered functional gradients in DS were correlated with the depression symptoms and receptor density of 5-HT2a, which was different from the NDS that related receptor of D1/2. This finding suggested that functional gradient changes may provide the potential biological significance of DS and NDS. For DS patients, the first explanation was that the related negative symptoms in VAN, and the second was that DS-introduced functional gradient changes were spatially positively correlated with 5-HT2a, especially the decreased functional gradient of LIB accompanied with the lower receptor density of 5-HT2a may suggest the absence of positive symptoms in DS patients. For NDS patients, their introduced functional gradient changes were negatively correlated with D1/2, the more decreased functional gradient of most networks with the higher receptor density of D1/2. These changes may be consistent with the dopamine hypothesis 2.Conclusion
Our findings suggest that brain network hierarchy may capture the distinct and common disruptions of DS and NDS as well as bridge the gap between macroscopic neuroimaging and molecular information. Overall, this study contributes to an integrative understanding of the mechanism of DS and NDS and further provide help with treatment.Acknowledgements
NoneReferences
1. Carpenter WT, Jr., Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
2. Howes OD, McCutcheon R, Owen MJ, Murray RM. The Role of Genes, Stress, and Dopamine in the Development of Schizophrenia. Biol Psychiatry. 2017;81:9-20.
3. Vos de Wael R, Benkarim O, Paquola C, Lariviere S, Royer J, Tavakol S, et al. BrainSpace: a toolbox for the analysis of macroscale gradients in neuroimaging and connectomics datasets. Communications biology. 2020;3:103.
4. Dukart J, Holiga S, Rullmann M, Lanzenberger R, Hawkins PCT, Mehta MA, et al. JuSpace: A tool for spatial correlation analyses of magnetic resonance imaging data with nuclear imaging derived neurotransmitter maps. Hum Brain Mapp. 2021;42:555-566.
Figures