4768
Regional dynamic brain function changes in adults with autism spectrum disorder1Department of Medical Imaging, Henan Provincial People’s Hospital, ZhengZhou, China, 2Department of Rehabilitation Medicine, Henan Provincial People’s Hospital, ZhengZhou, China, 3MR Collaboration, Siemens Healthineers Ltd., Beijing, China, 4Laboratory of Brain Science and Brain-Like Intelligence Technology, Institute for Integrated Medical Science and Engineering, ZhengZhou, China
Synopsis
Keywords: Psychiatric Disorders, fMRI (resting state)
Most neuroimaging studies investigating autism spectrum disorder (ASD) have focused on static brain function and used children as research subjects. However, this study investigated dynamic changes of regional neural function in adult ASD patients. Significant differences in dynamic regional homogeneity (dReHo) and dynamic amplitude of low-frequency fluctuation (dALFF) were observed based on resting state fMRI in several brain areas, such as the left middle/inferior temporal gyrus and left middle occipital gyrus. A significant correlation was found between clinical scores and the dReHo/dALFF values. These results suggested that dynamic regional brain function might be helpful in understanding neural mechanisms in ASD .Introduction
Autism spectrum disorder (ASD) is a neurodevelopmental condition with various causes and an unclear pathogenesis. Currently, resting-state functional magnetic resonance imaging (rs-fMRI) has become an essential technique for non-invasively studying neural mechanisms in ASD1. The amplitude of low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) are important indicators for understanding regional brain function. ReHo describes the consistency of activity in adjacent voxel areas, whereas ALFF shows the intensity of activity in a single voxel region. Previous studies1 revealed that ASD patients have abnormalities in ALFF and ReHo compared with healthy control subjects. However, most of the current rs-fMRI studies on ASD focus on the static characteristics of human brain functions, ignoring the dynamic features of spontaneous human brain activities in the temporal dimension. For example, one study has shown that the human brain could respond to internal or external stimuli through dynamic integration and adjustment on several time scales2. In recent years, researchers have begun to explore the dynamic mechanism of human spontaneous brain activity using the sliding window method3. This method allowed us to explore the dynamic changes of brain regional neuroimaging biomarkers. In contrast, as children with ASD mature, their brain function changes, and adults with ASD have distinctive features4. Therefore, studying neural mechanism changes in adult ASD patients is conducive to a comprehensive understanding of them and provides a theoretical basis for intervention and treatment in adult ASD patients. We used a sliding window technology combined with the dynamic ALFF (dALFF) and dynamic ReHo (dReHo) methods to explore the dynamic features of regional neural function for adult ASD patients. In addition, the relationships between the abnormal indexes (dALFF and dReHo) and the ASD clinical assessment index were investigated by correlation analyses. This study could enhance our perception of the neural mechanisms of adult ASD patients.Methods
This study included 77 adult ASD patients and 76 healthy controls (HCs) from the Autism Brain Imaging Data Exchange (ABIDE) database (http://fcon_1000.projects.nitrc.org/indi/abide/). Resting-state functional magnetic resonance imaging (rs-fMRI) and T1-weighted images from each subject were used for analysis. The Data Processing Assistant for Resting-State fMRI Analysis Toolkit (DPARSF, http://www.rfmri.org/DPARSF) was used to preprocess rs-fMRI data. We used the sliding window research approach to perform the dALFF and dReHo analyses based on Temporal Dynamic Analysis (TDA) toolkits in Data Processing and Analysis for Brain Imaging (DPABI) software. In our work, the window length was set at 30 TRs (60s), and the step length of the slide was set at 1 TR (2s). To obtain normalized dALFF and dReHo maps, the standard deviation (SD) of ReHo and ALFF values across all windows was initially calculated. Then, the SD ReHo and ALFF values were divided by the mean ReHo and ALFF values of all windows. Finally, we used an isotropic Gaussian kernel of 6 mm full-width-at-half-maximum (FWHM) to smooth the dynamic maps. Given the multiple pairwise comparisons, we chose a Gaussian random field (GRF) correction. A statistical significance level was employed with a voxel threshold of P < 0.001 and a cluster threshold of P < 0.05. Spearman correlation analyses were conducted in adult ASD patients between ASD clinical assessment scores and dReHo/dALFF values in brain regions exhibiting group differences.Results
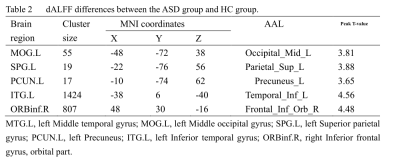
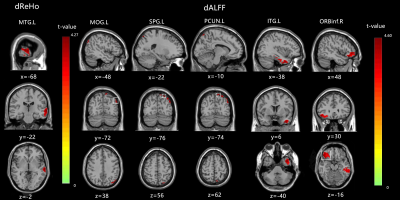
In the ASD group, significant differences in dReHo were observed in the left middle temporal gyrus (MTG.L). In addition, we found increased dALFF in the left middle occipital gyrus (MOG.L), left superior parietal gyrus (SPG.L), left precuneus (PCUN.L), left Inferior temporal gyrus (ITG.L), and right inferior frontal gyrus, orbital part (ORBinf.R), as shown in Figure1. Furthermore, a significant positive correlation was found between dALFF in the PCUN.L and the ADOS_TOTAL scores and ADOS_SOCIAL scores. In addition, for the dALFF in the ITG.L, SPG.L was positively associated with the ADOS_SOCIAL scores.Discussion & Conclusion
In our study, dynamic regional neural functions between adult ASD patients and healthy controls were conducted by sliding window analysis. Increased dReHo was found in the middle temporal gyrus (MTG), and increased dALFF was seen in the middle occipital gyrus (MOG), superior parietal gyrus (SPG), precuneus, inferior temporal gyrus, and inferior frontal gyrus, orbital part. Furthermore, correlation analyses showed that the ADOS scores were related to abnormal dALFF values in the precuneus, superior parietal gyrus, and inferior temporal gyrus in adult ASD patients. As the critical areas in the “social brain” network and visual center, MTG and MOG have been reported to be related to ASD patients5. From the perspective of temporal variability, our research reconfirmed that the MTG and MOG played an essential role in brain functional changes for adult ASD patients. Studies have shown that the SPG and precuneus were also brain regions associated with ASD patients6. This finding is consistent with our findings and further confirms that abnormal SPG and precuneus neural activity are neural mechanisms of abnormal social attributes in ASD patients. Detecting dynamic neural features in adult ASD patients could enhance our understanding of ASD-related neural mechanisms.Acknowledgements
No acknowledgementsReferences
1.Paakki JJ, Rahko J, Long X, et al. Alterations in regional homogeneity of resting-state brain activity in autism spectrum disorders. Brain research, 2010; 1321: 169-79.
2.Hutchison RM, Womelsdorf T, Allen EA, et al. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage, 2013; 80: 360-78.
3.Voytek B, Knight RT. Dynamic network communication as a unifying neural basis for cognition, development, aging, and disease. Biological psychiatry, 2015; 77 (12): 1089-97.
4.Justus SA, Powell PS, Duarte A. Intact context memory performance in adults with autism spectrum disorder. Sci Rep, 2021; 11 (1): 20482.
5.Xu J, Wang C, Xu Z, et al. Specific Functional Connectivity Patterns of Middle Temporal Gyrus Subregions in Children and Adults with Autism Spectrum Disorder. Autism research : official journal of the International Society for Autism Research, 2020; 13 (3): 410-22.
6.Travers BG, Kana RK, Klinger LG, Klein CL, Klinger MR. Motor learning in individuals with autism spectrum disorder: activation in superior parietal lobule related to learning and repetitive behaviors. Autism research : official journal of the International Society for Autism Research, 2015; 8 (1): 38-51.
Figures