4767
Assessing the Effect of Selective Serotonin Reuptake Inhibitor on GABA and Neurometabolic Levels in the Brains of Violent Offenders: A Pilot Study1School of Biomedical Sciences and Pharmacy, College of Health, Medicine and Wellbeing, University of Newcastle, Callaghan, Australia, 2Hunter Medical Research Institute, New Lambton Heights, Australia, 3School of Population Health, UNSW, Sydney, Australia, 4Research Imaging New South Wales, Division of Research & Enterprise, UNSW, Sydney, Australia, 5School of Medicine and Public Health, College of Health, Medicine and Wellbeing, University of Newcastle, Callaghan, Australia, 6School of Health Sciences, College of Health, Medicine and Wellbeing, University of Newcastle, Callaghan, Australia
Synopsis
Keywords: Psychiatric Disorders, Brain
Despite GABA implication in the regulation of aggression, the effects of selective serotonin reuptake inhibitor (SSRI) on GABA and Glx in an impulsive-aggressive population is yet to be explored. Seven repeat-offenders were treated with Sertraline and underwent MRI/MRS scans pre- and post-treatment (3-4 weeks of treatment). No significant changes (p≥0.05) in ACC-GABA+ or ACC-Glx were reported. Significant reduction in ACC-Glx/tCr (p=0.02) and increase in ACC-NAA (p=0.05) were found, while MM09+L09 increase approached significance (p=0.06). Further investigation is warranted. SSRIs clearly exert secondary effects on Glx levels in the ACC, with possible effects on NAA and MM09+L09.Background
A significant percentage of the prison population have a history of violent offences, including domestic violence1. Violence is one of the leading causes of death worldwide for people between 15-44 years old1. There is evidence of a strong association between poor impulse control (impulsivity) and violent offences2. A complex relationship exists between impulsivity, serotonin and gamma-aminobutyric acid (GABA) which is a primary neurotransmitter in the brain2,3. Treatment with selective serotonin reuptake inhibitors (SSRIs) in aggressive-impulsive populations has demonstrated a reduction in hostility and impulsivity4-6. Previous studies identified disrupted GABA signalling as a possible mechanism underlying the top-down regulation of aggression. The downstream effects of SSRIs on GABA and glutamine+glutamate (Gln+Glu, Glx) have not yet been explored in a population of violent offenders. In this study, we used both magnetic resonance spectroscopy (MRS) and MRS-edit techniques to investigate the complex relationship between serotonin, GABA, and Glx in the anterior cingulate cortex (ACC) due to its involvement in cognitive functions and association with aggression/impulsivity7.Materials and Methods
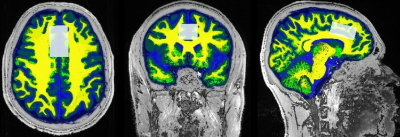
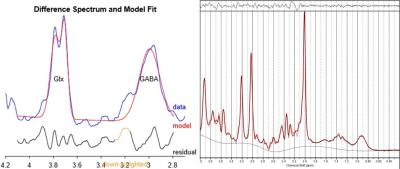
A total of 7 offenders over the age of 18 were treated with Sertraline (Setrona) and selected to participate in this sub-study. All MRI/MRS scans were undertaken on a 3T (Prisma, Siemens) MRI scanner equipped with a 64-channel coil. MRI/MRS data was acquired from the offender cohort at 2 timepoints; baseline (BL, i.e. before commencement of Sertraline) and 3-4 weeks follow-up (FU) of the SSRI intervention. Structural imaging using 3D T1-MPRAGE (TR/TE/TI=2000/3.5/1100ms, FOV=256x256mm2, voxel size:1mm3) as well as 3D T2-FLAIR (TR/TE/TI=5000/386/1800ms, FOV=256x256 mm2, voxel size: 1mm3) were acquired. H-MRS was applied using a Point RESolved Spectroscopy (PRESS) sequence acquired from the anterior cingulate cortex (ACC) (Figure 1) with the following acquisition parameters: TR/TE=2000/30ms, ACC voxel size =40x25x20 mm3, averages=128 with water suppression. Lastly, the parameters used for the MEscher-Garwood PRESS (MEGA-PRESS)8,9 scans were as follows, TR/TE=2000/68 ms, bandwidth=1200Hz, averages=128. FSL and SPM12 were used for total brain volume, grey matter (GM), white matter (WM), CSF volumes and segmentation of the MRS voxels10,11 (Figure 1). MRS data was exported offline and underwent post-processing using two methods, Gannet (analysis of GABA and Glx concentrations) and LCModel (analysis of major metabolites). Volumetric measures and the level of significant change in metabolite levels over 4 weeks were assessed using repeated measures ANOVA, with age and sex as covariates, followed by post hoc testing using Bonferroni.Results
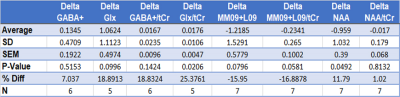
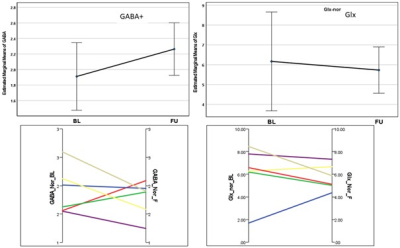
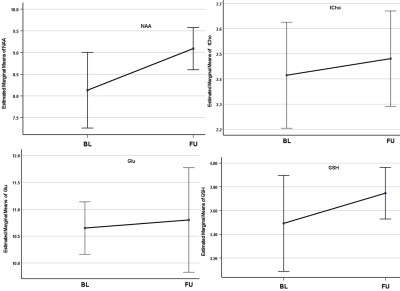
The results of the MEGA-PRESS (for GABA+(co-edited contributions from macromolecules and homocarnosine) and Glx) and PRESS (for N-acetylaspartate (NAA) and macromolecule 9+lipid9 (MM09+L09)) are summarised in Table 1 and Figure 2. An insignificant reduction of 7% in GABA+ level (p=0.52) and 18.8% in the GABA+ ratio with creatine (GABA+/tCr) (p=0.14) in the ACC was observed after 4 weeks of SSRI treatment. The average change in Glx and Glx/tCr showed 18.9% (p=0.09) and 25.4% (p=0.02) reductions at follow-up respectively. The variance of GABA+ and Glx concentrations between both timepoints is shown in Figure 3. Furthermore, the average absolute NAA concentration showed a significant increase of 10.6% (p=0.049) following the intervention, and the average change in MM09+L09/tCr approach significance, having increased by 16.9% (p=0.058) at follow-up. The changes in total choline (tCho), myo-inositol (Ins), Glu and NAA also show significant differences (p<0.05) between subjects. Lastly, the average change in absolute tCho, GSH, Glu and NAA concentrations from BL to FU is shown in Figure 4.Discussion
The primary findings of our study confirm the significant reduction of ACC-Glx with no significant change in ACC-GABA+ following SSRI treatment. These findings are in line with other studies12, that report a reduction in hippocampal Glx/tCr within healthy subjects, accompanied by no significant change in GABA+/tCr. Additional reports also suggest that the occipital cortex (OCC) may be particularly susceptible in its ability to reflect changes in GABA due to SSRI intervention, independent of clinical changes in mood13,14. Bhagwagar15 reported increased GABA levels in a cohort of healthy participants following SSRI administration, compared to the administration of saline, in the OCC. Current literature also suggests that SSRI treatment only acts to normalise clinically low pre-treatment GABA concentrations. Our findings demonstrate that there was a significant increase in NAA (neuronal marker) and tCho levels over the time following SSRI treatment. tCho results agree with another study16 which showed significant increases in Cho/Cr in the ventral prefrontal white matter of major depressive disorder (MDD) patients, however, no change was reported for NAA as they reported no significant change over time.Conclusion
The results of this pilot study warrant further investigation via a longitudinal study, of the secondary effects of SSRIs on GABA+ and Glx in the ACC of impulsive-violent offenders that are at a high risk of reoffending. The findings so far clearly indicate that SSRI treatment exerts secondary effects on Glx concentrations in the ACC, with additional possible effects on NAA and MM09+L09.Acknowledgements
The authors acknowledge the facilities and scientific and technical assistance of the National Imaging Facility, a National Collaborative Research Infrastructure Strategy (NCRIS) capability, at the Hunter Medical Research Institute Imaging Centre, University of Newcastle, and Research Imaging NSW, UNSW Sydney.References
1. Bricknell S. Trends in violent crime: Australian Institute of Criminology Canberra: 2008.
2. Narvaes R, Almeida R. Aggressive behavior and three neurotransmitters: Dopamine, GABA, and serotonin—A review of the last 10 years. Psychology & Neuroscience 2014;7:601-607.
3. de Almeida RMM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: Dopamine, serotonin and GABA. European Journal of Pharmacology 2005;526(1):51-64.
4. Hrabovszky E, Halász J, Meelis W, Kruk MR, Liposits Z, Haller J. Neurochemical characterization of hypothalamic neurons involved in attack behavior: Glutamatergic dominance and co-expression of thyrotropin-releasing hormone in a subset of glutamatergic neurons. Neuroscience 2005;133(3):657-666.
5. Siever LJ. Neurobiology of Aggression and Violence. American Journal of Psychiatry 2008;165(4):429-442.
6. Li JN, Liu XL, Li LA-OX. Prefrontal GABA and glutamate levels correlate with impulsivity and cognitive function of prescription opioid addicts: A (1) H-magnetic resonance spectroscopy study. (1440-1819 (Electronic)).
7. Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: a neuroanatomical correlate of aggression and defiance in boys. Behav Neurosci 2008;122(3):677-684.
8. Fuchs VR, Sox HC, Jr. Physicians' views of the relative importance of thirty medical innovations. (0278-2715 (Print)).
9. Harris AD, Saleh MG, Edden RA. Edited (1) H magnetic resonance spectroscopy in vivo: Methods and metabolites. (1522-2594 (Electronic)).
10. Quadrelli S, Mountford C, Ramadan S. Hitchhiker's Guide to Voxel Segmentation for Partial Volume Correction of In Vivo Magnetic Resonance Spectroscopy. Magn Reson Insights 2016;9:1-8. 11. Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 2004;23:S208-S219.
12. Spurny B, Vanicek T, Seiger R, et al. Effects of SSRI treatment on GABA and glutamate levels in an associative relearning paradigm. NeuroImage 2021;232:117913.
13. Sanacora G, Mason GF, Rothman DL, Krystal JH. Increased Occipital Cortex GABA Concentrations in Depressed Patients After Therapy With Selective Serotonin Reuptake Inhibitors. American Journal of Psychiatry 2002;159(4):663-665.
14. Brennan BP, Admon R, Perriello C, et al. Acute change in anterior cingulate cortex GABA, but not glutamine/glutamate, mediates antidepressant response to citalopram. Psychiatry Res Neuroimaging 2017;269:9-16.
15. Bhagwagar Z, Wylezinska M, Taylor M, Jezzard P, Matthews PM, Cowen PJ. Increased Brain GABA Concentrations Following Acute Administration of a Selective Serotonin Reuptake Inhibitor. American Journal of Psychiatry 2004;161(2):368-370.
16. Zhang Y, Han Y, Wang Y, et al. A MRS study of metabolic alterations in the frontal white matter of major depressive disorder patients with the treatment of SSRIs. BMC Psychiatry 2015;15:99.
Figures