4763
HYPOTHALAMIC VOLUME IS ASSOCIATED WITH BODY MASS INDEX
Stephanie S. G. Brown1, Margaret Westwater2, Jakob Seidlitz3, Hisham Ziauddeen1, and Paul Fletcher1
1Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom, 2University of Oxford, Oxford, United Kingdom, 3University Pennsylvania, Philadelphia, PA, United States
1Department of Psychiatry, University of Cambridge, Cambridge, United Kingdom, 2University of Oxford, Oxford, United Kingdom, 3University Pennsylvania, Philadelphia, PA, United States
Synopsis
Keywords: Psychiatric Disorders, Segmentation, OBESITY
The hypothalamus plays a major role in appetite regulation via well-defined orexigenic and anorexigenic pathways. Perturbation of these pathways is known to alter feeding behaviour and body mass. Using an automated technique, we segmented the hypothalamus in 1299 young adults with variation in body mass index (BMI). We showed that in participants who were overweight or obese, the hypothalamus and constituent nuclei were significantly increased in volume compared to those of a healthy weight. Further, these volume increases are predominantly localised to the arcuate and paraventricular nuclei, which form the core of the neuroendocrine appetite maintenance system.Body of abstract
IntroductionThe hypothalamus is a major regulator of homeostatic processes. Key to this function, the hypothalamus maintains balance between energy expenditure and food intake, disruption of which results in over- or under-eating relative to bodily energy output 1. In turn, this energy imbalance leads to alterations in body mass, which can have significant and myriad health implications 2. The hypothalamus is located ventrally to the thalamus and forms part of the diencephalon 3. It connects extensively to cortical, subcortical and brainstem regions 4. There is abundant evidence that neuronal populations within the hypothalamus, located within the arcuate nucleus and the paraventricular nucleus (PVN), have major contributions to appetite regulation. The arcuate nucleus has been particularly well-characterised in its relation to appetite, with its two opposite acting distinct neuronal populations: the orexigenic neuropeptide Y/agouti-related peptide (AgRP) neurons and the anorexigenic pro-opiomelancortin (POMC) neurons 5, 6. The PVN, similarly to POMC neuronal function, is thought to also carry out anorexigenic signalling within the hypothalamus. The paraventricular and arcuate nuclei therefore comprise the predominant food-related homeostatic control centres of the hypothalamus, with disruption to any area of this internal circuitry resulting in marked changes to appetite and body mass. Much of the existing evidence for the role of the hypothalamus in appetite regulation originates from animal studies 7. This is partly due to difficulties in imaging and studying the hypothalamus in humans, including a lack of defined contrast in structural images in this area of the brain, meaning that the hypothalamus and its constituent functional nuclei are challenging to delineate. Some studies have, however, manually segmented this region in human MRI scans in vivo 3, 8. A major limitation of this approach is that manual tracing of the hypothalamus is time-consuming and can have low inter-rater reliability, and is therefore poorly suited for the large neuroimaging datasets that are required for adequate statistical power and result reproducibility. However, a recently developed algorithm, created using convolutional neural networks 9, now allows for automated segmentation of the hypothalamus with high reported accuracy, which presents the opportunity for larger-scale investigation into the hypothalamic structure in humans in vivo.
Methods
Participants and MRI acquisitionData for this study were drawn from four existing MRI datasets: Obese and overweight participants with a BMI over 24.9 (n = 65) 10, normal weight control participants matched to the obese/overweight group from the NeuroScience in Psychiatry Network U-Change project (n = 39) 11, participants with anorexia nervosa (AN) (n = 21), bulimia nervosa (BN) (n = 33) and age-matched controls (n = 30) 12, and the Human Connectome Project (HCP) Young Adult dataset (n = 1111) 13. MRI data analysis 3T T1-weighted acquisitions were pre-processed using the FreeSurfer v7.0 ‘recon-all’ pipeline (https://surfer.nmr.mgh.harvard.edu/fswiki/recon-all). Pre-processed structural data were then segmented to extract the whole hypothalamus and hypothalamic nuclei using an automated tool based on a deep convolutional neural network in FreeSurfer development version 7 9 (Fig. 1). Subnuclei per hemisphere were segmented and labelled as the following: anterior-inferior, anterior-superior, posterior, tubular inferior and tubular superior. Hypothalamic volume data was also normalised to intracranial volume (ICV) 14. Results Non-parametric t-testing and correction for multiple comparison using false discovery rate (FDR) revealed that both left and right whole hypothalamic structures were significantly increased in volume in the obesity group compared to their matched control group (pcorr < 0.05). While the AN group exhibited smaller hypothalami than their matched control group when volume was non-normalised to ICV, ICV normalisation removed this effect (Fig. 2). In the HCP-Young Adult dataset (total n = 1111), participants were grouped into underweight = BMI < 18.49 (n = 14), normal weight = BMI 18.5 – 24.9 (n = 474), overweight = BMI 25 – 29.9 (n = 375), obese = BMI 30 + (n = 242). For both the obese and overweight groups, whole hypothalamus and multiple hypothalamic nuclei demonstrated significantly increased volume compared to healthy weight participants (Fig. 3). The HCP-Young Adult whole hypothalamic data were regressed against BMI, showing a significant relationship (p < 0.05) between increasing hypothalamic size and increasing BMI (Fig. 4).
Discussion
Our initial findings from a relatively small dataset show that, in overweight or obese participants, the whole hypothalamic structure is significantly larger than in matched controls of a healthy weight. Further, we show that in AN, reduction in hypothalamus volume compared to matched controls is proportionate to ICV, and most likely a consequence of decreased overall brain size 15. In the larger HCP-Young Adult dataset, we replicated the key finding that in overweight/obese people (BMI > 24.9), hypothalamus volume is significantly increased. Specifically, the constituent nuclei segmentations reveal that regions containing the arcuate nucleus and PVN 9 are the areas responsible for overall hypothalamic volume increase. These regions are known to be the centres of endocrine appetite control 7.
Conclusion
We show here for the first time primary and replicated findings of increased hypothalamic volume in relation to higher than healthy BMI. This signals important structural changes to normal neuroendocrine appetite regulation, which may support future mechanisms of novel appetite suppression.
Acknowledgements
No acknowledgement found.References
1. King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221-44.2. Konturek PC, Konturek JW, Czesnikiewicz-Guzik M, Brzozowski T, Sito E and Konturek SJ. Neuro-hormonal control of food intake: basic mechanisms and clinical implications. J Physiol Pharmacol. 2005;56 Suppl 6:5-25.3. Neudorfer C, Germann J, Elias GJB, Gramer R, Boutet A and Lozano AM. A high-resolution in vivo magnetic resonance imaging atlas of the human hypothalamic region. Sci Data. 2020;7:305.4. Brown S. S. G. MK, Fletcher P., Holland A. J. . In-vivo neuroimaging evidence of hypothalamic alteration in Prader-Willi syndrome. . Brain Communications In Press. 2022.5. Bittel DC, Kibiryeva N, McNulty SG, Driscoll DJ, Butler MG and White RA. Whole genome microarray analysis of gene expression in an imprinting center deletion mouse model of Prader-Willi syndrome. Am J Med Genet A. 2007;143A:422-9.6. Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P and Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci. 2013;33:3624-32.7. Sohn JW. Network of hypothalamic neurons that control appetite. BMB Rep. 2015;48:229-33.8. Schindler S, Schonknecht P, Schmidt L, Anwander A, Strauss M, Trampel R, Bazin PL, Moller HE, Hegerl U, Turner R and Geyer S. Development and evaluation of an algorithm for the computer-assisted segmentation of the human hypothalamus on 7-Tesla magnetic resonance images. PLoS One. 2013;8:e66394.9. Billot B, Bocchetta M, Todd E, Dalca AV, Rohrer JD and Iglesias JE. Automated segmentation of the hypothalamus and associated subunits in brain MRI. Neuroimage. 2020;223:117287.10. Westwater ML, Vilar-Lopez R, Ziauddeen H, Verdejo-Garcia A and Fletcher PC. Combined effects of age and BMI are related to altered cortical thickness in adolescence and adulthood. Dev Cogn Neurosci. 2019;40:100728.11. Kiddle B, Inkster B, Prabhu G, Moutoussis M, Whitaker KJ, Bullmore ET, Dolan RJ, Fonagy P, Goodyer IM and Jones PB. Cohort Profile: The NSPN 2400 Cohort: a developmental sample supporting the Wellcome Trust NeuroScience in Psychiatry Network. Int J Epidemiol. 2018;47:18-19g.12. Westwater ML, Mancini F, Gorka AX, Shapleske J, Serfontein J, Grillon C, Ernst M, Ziauddeen H and Fletcher PC. Prefrontal Responses during Proactive and Reactive Inhibition Are Differentially Impacted by Stress in Anorexia and Bulimia Nervosa. J Neurosci. 2021;41:4487-4499.13. Strike LT, Hansell NK, Couvy-Duchesne B, Thompson PM, de Zubicaray GI, McMahon KL and Wright MJ. Genetic Complexity of Cortical Structure: Differences in Genetic and Environmental Factors Influencing Cortical Surface Area and Thickness. Cereb Cortex. 2019;29:952-962.14. Voevodskaya O, Simmons A, Nordenskjold R, Kullberg J, Ahlstrom H, Lind L, Wahlund LO, Larsson EM, Westman E and Alzheimer's Disease Neuroimaging I. The effects of intracranial volume adjustment approaches on multiple regional MRI volumes in healthy aging and Alzheimer's disease. Front Aging Neurosci. 2014;6:264.15. Walton E, Bernardoni F, Batury VL, Bahnsen K, Lariviere S, Abbate-Daga G, Andres-Perpina S, Bang L, Bischoff-Grethe A, Brooks SJ, Campbell IC, Cascino G, Castro-Fornieles J, Collantoni E, D'Agata F, Dahmen B, Danner UN, Favaro A, Feusner JD, Frank GKW, Friederich HC, Graner JL, Herpertz-Dahlmann B, Hess A, Horndasch S, Kaplan AS, Kaufmann LK, Kaye WH, Khalsa SS, LaBar KS, Lavagnino L, Lazaro L, Manara R, Miles AE, Milos GF, Monteleone AM, Monteleone P, Mwangi B, O'Daly O, Pariente J, Roesch J, Schmidt UH, Seitz J, Shott ME, Simon JJ, Smeets PAM, Tamnes CK, Tenconi E, Thomopoulos SI, van Elburg AA, Voineskos AN, von Polier GG, Wierenga CE, Zucker NL, Jahanshad N, King JA, Thompson PM, Berner LA and Ehrlich S. Brain Structure in Acutely Underweight and Partially Weight-Restored Individuals With Anorexia Nervosa: A Coordinated Analysis by the ENIGMA Eating Disorders Working Group. Biol Psychiatry. 2022;92:730-738.Figures
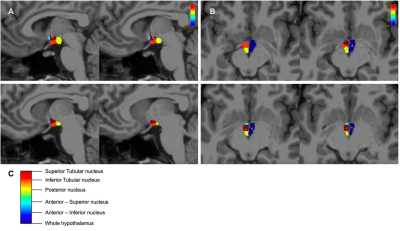
Figure 1. A Sagittal brain slices showing segmented hypothalamic nuclei B Axial brain slices showing segmented hypothalamic nuclei and whole hypothalamus.
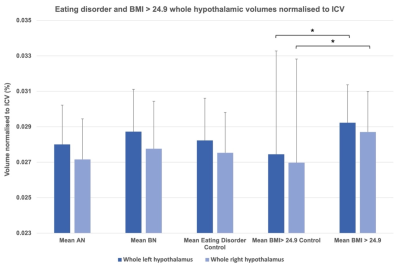
Figure 2. Anorexia nervosa (AN), bulimia nervosa (BN), eating disorder matched control, overweight/obese and overweight/obese matched control groups: mean whole hypothalamic volumes normalised to intracranial volume (ICV), error bars represent standard deviation. Whole left and whole right hypothalami were significantly larger in those with body mass index (BMI) greater than 24.9 compared to healthy weight controls.
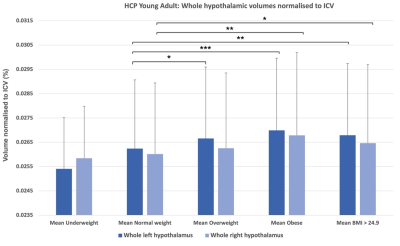
Figure 3. Mean whole hypothalamic volumes (left and right) and non-parametric t-test results from the HCP-Young Adult dataset (* = pcorr < 0.05, ** = pcorr < 0.01, *** = pcorr < 0.001). Error bars represent standard deviation.
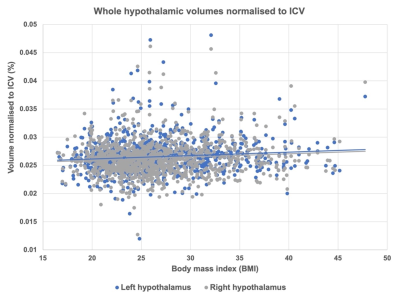
Figure 4. Whole hypothalamic volumes normalised to intracranial volume (ICV) (left and right) regressed in the HCP-Young Adult dataset against body mass index (BMI) showed a significant positive association between increasing BMI and increasing hypothalamic volume (p < 0.05, R2 = 0.01).
DOI: https://doi.org/10.58530/2023/4763