4755
A pilot study of brain glutamine levels in schizophrenia and control subjects: relationship to blood ammonia and cognition1JHU SOM, Baltimore, MD, United States, 2Obstetrics & Gynecology, Weill Cornell Medicine, New York, NY, United States
Synopsis
Keywords: Psychiatric Disorders, Psychiatric Disorders, 7T, MRS, Schizophrenia, Metabolism, Glutamine
It has previously been shown that brain glutamine (Gln) may be elevated is some patients with schizophrenia (Sz), and associated with cognitive deficits. Underlying causes of Gln elevation in Sz are unclear, but may be related to increased blood ammonia (NH3) levels. Brain Gln was measured in 12 subjects (5 Sz, 7 healthy controls (HC)) using 7T MR spectroscopy; brain Gln was found to correlate with blood NH3 levels (p < 0.05). and negatively correlate with measures of cognitive performance. This preliminary study therefore supports the hypothesis linking blood ammonia, brain Gln and cognitive performance.Introduction
Previous MR spectroscopy (MRS) studies have suggested abnormally elevated brain glutamine (Gln) levels in patients with chronic schizophrenia (Sz) (1,2). The underlying cause of this metabolic abnormality is unclear, but one possibility is that it may be driven by increased blood ammonia levels, since Gln is synthesized in the brain from glutamate (Glu) and NH3 (3). Blood ammonia levels are commonly related to liver function, and, via their effects on the brain, may be one factor contributing to cognitive impairment and negative symptom severity in patients with schizophrenia, perhaps due to perturbed Glu-Gln cycling.In this interim analysis of an ongoing study, Gln was measured in multiple brain regions using 7T MRS, and compared to both concurrent blood NH3 levels and scores on a comprehensive battery of neuropsychological tests (4).Methods
To date, 12 subjects have completed all study procedures (5 Sz, 7 HC, 4M/8F, age 34 ± 9 years). There were 2 study visits, one for consent, screening and detailed neuropsychological assessments (4), and a 2nd visit within 7 days for blood draw (NH3 determination) and 7T MRI. Sz subjects were diagnosed using the DSM-5 criteria, were clinically stable for at least 4 weeks, and were restricted to those treated with 2nd generation antipsychotic medication. Exclusion criteria for all subjects were recent substance dependence or use of valproate, regular benzodiazepine use, history of diabetes, liver disease, and obesity (BMI ≥ 35).All participants were scanned using a 7T scanner (Philips ‘Achieva’, Best, Netherlands) equipped with a 32-channel receive head coil. 3D T1-weighted high resolution anatomical images were acquired using an MPRAGE sequence. Spectra were recorded from anterior cingulate cortex (ACC; 30×20×20 mm3), left dorsolateral prefrontal cortex (DLPFC; 25×20×20 mm3) left centrum semiovale (CSO; 40×20×15 mm3), bilateral thalamus (Thal, 20×30×15 mm3), and left hippocampus (hippo; 35×15×15 mm3), using a STEAM sequence (TR/TE/TM = 3000/14/33 ms, 64 NEX, 4m 24s per VOI). VAPOR water suppression and 2nd order shim-correction used. In addition, a scan without water suppression was also acquired from each voxel (NEX = 2).Water-scaling and eddy-current correction were applied using the unsuppressed water signal. Spectra were analyzed in the ‘LCModel’ software package (5) using a basis set containing 20 metabolites as described previously (4). Cramér-Rao lower bounds (CRLBs) were calculated. Statistical analyses were performed with ‘R’ (version 3.5.3). Two-sided Pearson correlation analysis was conducted to examine the potential correlation between the blood NH3 levels and brain Gln and (and Gln/Glu ratios) and cognitive performance, with the levels of significance set at p < 0.05.Results
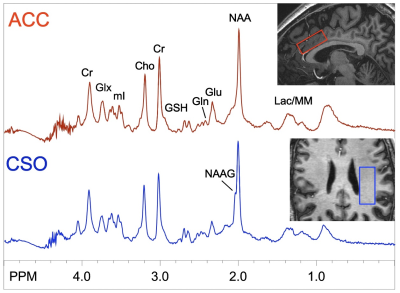
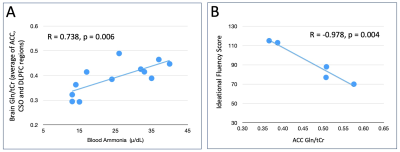
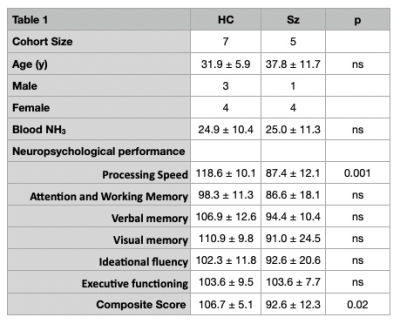
Table 1 shows demographics of the subjects, and summary of blood NH3 levels and neuropsychological test scores. As expected, patients performed significantly worse on overall neuropsychological tests, particularly in processing speed and overall composite score. Figure 1 shows representative spectra from 2 selected brain regions (ACC, CSO) in one subject with schizophrenia. No significant differences in Gln (expressed as concentrations, or as Gln/Glu) were found between groups (p > 0.05).In HC, significant correlations were found between Gln levels (in the ACC and DLPFC) and blood NH3 levels. In the combined HC and Sz population, Gln levels in ACC, DLPFC and hippocampus correlated with blood NH3 levels. Indices of average brain Gln levels also correlated with blood NH3 (all p< 0.05, Figure 2A).In the Sz group only, significant inverse correlations were found between ACC and thalamic Gln and ideational fluency and executive function scores (p < 0.05, Figure 2B); however, no significant correlations were found between blood NH3 levels and cognitive performance scores in either HC, Sz or the combined group.Discussion
In this preliminary study of subjects without any significant liver dysfunction, brain glutamine levels and blood NH3 levels were found to have significant correlations, supporting the hypothesis that blood NH3 levels are an important factor in brain Gln synthesis.With the current sample size, no significant correlations were found between cognitive scores and blood NH3 levels. Lower performance in ideational fluency and executive function was associated with higher ACC and thalamic Gln. This is consistent with the hypothesis that increasing Gln levels are associated with worsening cognitive performance, which was significantly worse in Sz compared to controls. Overall, these preliminary results support the hypothesis linking blood NH3, brain Gln, and cognitive performance. Data collection from a larger cohort of subjects is planned.Acknowledgements
Supported by NIH R21MH127285.References
1. Bustillo JR, Chen H, Jones T, Lemke N, Abbott C, Qualls C, Canive J, Gasparovic C. Increased glutamine in patients undergoing long-term treatment for schizophrenia: a proton magnetic resonance spectroscopy study at 3 T. JAMA Psychiatry 2014;71(3):265-272.
2. Wijtenburg SA, Wang M, Korenic SA, Chen S, Barker PB, Rowland LM. Metabolite Alterations in Adults With Schizophrenia, First Degree Relatives, and Healthy Controls: A Multi-Region 7T MRS Study. Front Psychiatry 2021;12:656459.
3. Kreis R, Ross BD, Farrow NA, Ackerman Z. Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR spectroscopy. Radiology 1992;182(1):19-27.
4. Wang AM, Pradhan S, Coughlin JM, Trivedi A, DuBois SL, Crawford JL, Sedlak TW, Nucifora FC, Jr., Nestadt G, Nucifora LG, Schretlen DJ, Sawa A, Barker PB. Assessing Brain Metabolism With 7-T Proton Magnetic Resonance Spectroscopy in Patients With First-Episode Psychosis. JAMA Psychiatry 2019;76(3):314-323.
5. Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30(6):672-679.
Figures