4751
Microstructural Alterations in Schizophrenia evaluated by Multidimensional Diffusion MRI and Inhomogeneous Magnetization Transfer Imaging
Khin Khin Tha1, Maho Kitagawa2, Samo Lasic3, Karin Bryskhe3, Jihun Kwon4, Marteen Versluis5, and Naoki Hashimoto6
1Global Center for Biomedical Science and Engineering, Hokkaido University Faculty of Medicine, Sapporo, Japan, 2Laboratory for Biomarker Imaging Science, Hokkaido University Graduate School for Biomedical Science and Engineering, Sapporo, Japan, 3Random Walk Imaging, Lund, Sweden, 4Philips Japan, Tokyo, Japan, 5Philips Healthcare, Best, Netherlands, 6Department of Psychiatry, Hokkaido University Faculty of Medicine, Sapporo, Japan
1Global Center for Biomedical Science and Engineering, Hokkaido University Faculty of Medicine, Sapporo, Japan, 2Laboratory for Biomarker Imaging Science, Hokkaido University Graduate School for Biomedical Science and Engineering, Sapporo, Japan, 3Random Walk Imaging, Lund, Sweden, 4Philips Japan, Tokyo, Japan, 5Philips Healthcare, Best, Netherlands, 6Department of Psychiatry, Hokkaido University Faculty of Medicine, Sapporo, Japan
Synopsis
Keywords: Psychiatric Disorders, Diffusion/other diffusion imaging techniques, schizophrenia, multidimensional diffusion
This prospective study aimed to identify the role of multidimensional diffusion (MDD) MRI and inhomogeneous magnetization transfer (ihMT) imaging, the former reported as capable of quantifying various microstructural diffusion properties and differentiating tissue-specific subparts and the latter as selective to changes in myelin, in underpinning the microstructural brain changes in schizophrenia. Alterations in MDD MRI indices were observed, associated with negative symptoms of schizophrenia. Altered size and shape of cells other than myelin (probably glia or neurons) are thought as coined in the pathophysiological mechanisms.Background and Purpose
Schizophrenia is a severe psychiatric disorder characterized by delusion and hallucination, affecting approximately 0.30–0.66% of the population1. MRI has been extensively used to study the underlying structural and microstructural brain changes. Aberrant functioning of cortical microcircuits and altered white matter (WM) microstructural properties have been reported, but information about tissue- or cell-type-specific changes is still insufficient2.This prospective study aimed to identify the role of multidimensional diffusion (MDD) MRI and inhomogeneous magnetization transfer (ihMT) imaging, the former reported as capable of quantifying various microstructural diffusion properties and differentiating tissue-specific subparts3 and the latter as selective to changes in myelin4, in underpinning the microstructural brain changes in schizophrenia.
Methods
This prospective study was approved by the local institutional review board. Eighteen schizophrenic patients (10 men; mean age = 33.7 ± 8.6 years) who consented to the study were serially enrolled. Twenty age and sex-matched healthy subjects (10 men; mean age = 32.1 ± 9.5 years) were also recruited as the control group. All participants underwent brain MRI using a 3T scanner and 32-channel head coil (Ingenia Elition X, Philips Healthcare). The scan protocol included MDD MRI (TR/TE = 4500/ 127 ms, 59 linear, 33 spherical, and 32 planar b-tensors in an interleaved fashion using optimized gradient waveforms for b = 100, 700, 1400, 2000 s mm-2), ihMT imaging {TR/TE/ΔTE = 115/3.5/5.7 ms, 6 Hann-shaped RF pulses (pulse duration = 0.9 ms, frequency offset (Δf) = ±7 kHz)}, and 3D-T1WI (TR/TE = 7.1/3.2 ms, FA = 8°).From the MDD MRI images, whole-brain fractional anisotropy (FA), mean diffusivity (MD), anisotropic mean kurtosis (MKA), isotropic mean kurtosis (MKI), and microscopic fractional anisotropy (μFA) were calculated voxel-by-voxel (dVIEWR, MICE ToolkitTM; Random Walk Imaging AB and NONPI Medical AB, Sweden). Similarly, from the ihMT images, ihMT ratio (ihMTR) was calculated using the formula from Ref (4). From 3D-T1WI, the gray matter (GM), WM, and cerebrospinal fluid (CSF) volumes were calculated.
FA, MD, MKA, MKI, μFA, and ihMTR were compared between the two groups using two-sample t-tests. Uncorrected P<0.001 for clusters exceeding 25 voxels was considered statistically significant. The GM, WM, and CSF volumes were also compared using two-sample t-tests. For this purpose, P<0.05 was considered statistically significant. Significant clusters, if any, were tested for correlation with the positive and negative syndrome scale (PANSS), the brief negative symptom scale (BNSS), and total disease duration using Pearson's product-moment correlation analysis. Considering multiple comparisons, P<0.01 was taken as statistically significant. To identify tissue-specific subparts responsible for the MDD MRI changes, the mean (E) and variance (V) of tensor size ([Diso]) and shape ([DΔ2]) of significant clusters were also extracted.
Results
The schizophrenic patients had clusters with a significant decrease in FA, MKA, and μFA, and a significant increase in MD, compared to healthy controls (Fig 1 and 2). There was no significant difference in MKI, ihMTR, and the GM, WM, and CSF volumes between the two groups.Further analysis of the clusters with significant alterations revealed a significant difference in E and V of Diso and DΔ2 between the two groups, suggesting a perturbed size-shape space relationship in the patients (Fig 3). In particular, these significant clusters had a lower E of [DΔ2] and higher E of [Diso] for isotropic diffusion environments with relatively low diffusivity (localized in the lower left half part when the diffusion tensor distribution was divided into three bins based on [DΔ2] and[Diso]) and lower E and V of [Diso] and lower E of [DΔ2] for signal fractions associated with more elongated cells (the upper left half part of the bins).
Of significant clusters, the lower the FA in the right thalamus (MNI coordinates = 15.8 -23.3 -0.5) the more difficult was in abstract thinking (r=-0.659, P=0.008), and the lower the MKA in the corpus callosum (MNI coordinates = -10 42 4), the poorer the rapport (r=-0.702, P=0.004). The μFA in the left cerebellum (MNI coordinates = -30 -54 -48) had a moderate positive correlation with unusual thought content (r=0.663, P=0.007) items of the PANSS (Fig 4).
Discussion
The clusters with significant microstructural alterations are located in the sites that are known to be occasionally involved in schizophrenia. These sites have been considered responsible for the observed symptoms (e.g., cerebellar involvement can cause cognitive dysmetria, which is general dyscoordination of sensorimotor and mental processes5). Lacking a change in ihMTR and the brain volumes suggests that myelin is not primarily affected and the brain volume may be preserved until late. In other words, alterations in the microstructural environment are probably mainly due to alterations in the architecture (i.e., size and shape) of the glia or neurons. The observation of a correlation with negative symptoms of PANSS suggests that the microstructural alterations are the basis of negative symptoms and treatment targeting these alterations may alleviate these symptoms.Conclusion
This study evaluated the microstructural brain alterations in schizophrenia, using MDD MRI and ihMT imaging. MDD MRI may be useful in identifying the microstructural brain changes, including perturbations in the size-shape space of diffusion tensor distributions, and explaining the negative symptoms of schizophrenia.Acknowledgements
No acknowledgement found.References
- Narita H, Tha KK, Hashimoto N, Hamaguchi H, Nakagawa S, Shirato H, Kusumi I. Mean kurtosis alterations of cerebral white matter in patients with schizophrenia revealed by diffusion kurtosis imaging. Prog Neuropsychopharmacol Biol Psychiatry 2016: 71; 169-175.
- Carreira Figueiredo I, Borgan F, Pasternak O, Turkheimer FE, Howes OD. White-matter free-water diffusion MRI in schizophrenia: a systematic review and meta-analysis.Neuropsychopharmacology 2022; 47: 1413-1420.
- Naranjo ID, Reymbaut A, Brynolfsson P, Lo Gullo R, Bryskhe K, Topgaard D, Giri DD, Reiner JS, Thakur SB, Pinker-Domenig K. Multidimensional Diffusion Magnetic Resonance Imaging for Characterization of Tissue Microstructure in Breast Cancer Patients: A Prospective Pilot Study.Cancers (Basel) 2021; 31: 1606.
- Chen G, Fu S, Chen P, Zhong S, Chen F, Qian L, Luo Z, Pan Y, Tang G, Jia Y, Huang L, Wang Y. Reduced myelin density in unmedicated major depressive disorder: an inhomogeneous magnetization MRI study. J Affect Disord 2022; 300: 114-120.
- Picard H, Amado I, Mouchet-Mages S, Olié JP, Krebs MO. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophr Bull 2008; 34: 155–172.
Figures
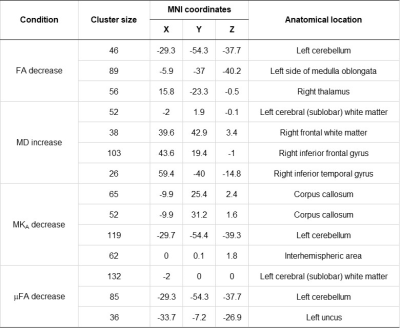
Fig 1. Clusters with a significant difference in fractional anisotropy (FA), mean diffusivity (MD), anisotropic mean kurtosis (MKA), and microscopic FA (μFA) in schizophrenic patients. Uncorrected P<0.001 for clusters containing at least 25 voxels are listed.
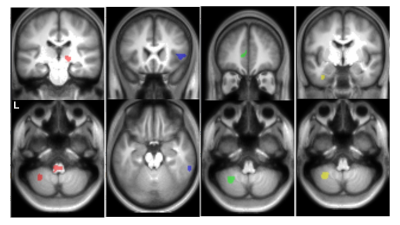
Fig 2. Examples of clusters with significant alterations in
schizophrenic patients (uncorrected P<0.001 for clusters containing at least
25 voxels) are shown. From your left are the clusters with FA decrease (red
voxels), MD increase (blue voxels), MKA decrease (green voxels), and μFA decrease
(yellow voxels) overlaid on a T1-weighted image template. L indicates left.
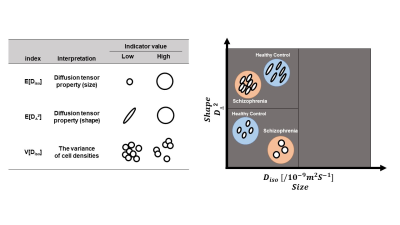
Fig 3. Binning of the size-shape space of diffusion tensor distributions in schizophrenic patients. The results are shown in comparison with the healthy subjects. The schizophrenic patients had lower E of [DΔ2] and higher E of [Diso] for isotropic diffusion environments with relatively low diffusivity (the lower left half part of the three bins) and lower E and V of [Diso] and lower E of [DΔ2] for signal fractions associated with more elongated cells (the upper left half part of the three bins)3.
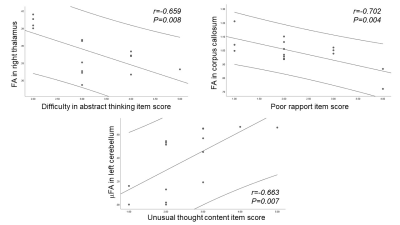
Fig 4. Scatterplots showing significant correlations between the MDD MRI indices (i.e., FA, MKA, and μFA) and the negative symptoms of PANSS. The mean and 95% confidence interval (CI) are given.
DOI: https://doi.org/10.58530/2023/4751