4732
Deep learning-based image quality and spatial resolution improvement in Turbo Spin Echo Diffusion Weighted Imaging for prostate1Philips Japan, Tokyo, Japan, 2Tokyo Metropolitan Police Hospital, Nakano, Japan, 3Philips Healthcare, Best, Netherlands
Synopsis
Keywords: Prostate, Diffusion/other diffusion imaging techniques
Diffusion-weighted imaging (DWI) plays an important role in assessing the significance of prostate cancer. DWI with Turbo Spin Echo readout (TSE-DWI) is robust to image distortion but suffers from low signal to noise ratio. In this study, we investigated the use of prototype AI-based reconstruction technique (SmartSpeed Precise Image) to improve the image quality of TSE-DWI images. The image quality was compared between conventional Compressed-SENSE (C-SENSE), SmartSpeed AI, and SmartSpeed Precise Image. Volunteer data demonstrated a significant improvement of sharpness in both b=0 and 1000 s/mm2 images as well as ADC map, compared with C-SENSE and SmartSpeed AI reconstructions.
Introduction
The multiparametric MRI approach is commonly taken for diagnosing and staging prostate cancer patients1. Among multiparametric MRI, diffusion-weighted imaging (DWI), including apparent diffusion coefficient (ADC) map derived from DWI, plays an important role in assessing the clinical significance of prostate cancer2,3. For prostate DWI, Echo Planar Imaging (EPI)-DWI is generally used due to its high signal to noise ratio (SNR)3,4. However, EPI-DWI is sensitive to susceptibility differences and can cause artifactual geometric distortion in the proximity to air (Figure 1a and b)5. This is relevant particularly for patients with hip implants6. Turbo Spin Echo (TSE), another readout method for DWI, is more robust to such distortion, but suffers from low SNR compared with EPI-DWI (Figure 1c)7,8.Recently there has been a growing interest in using Artificial Intelligence (AI) to improve the image quality of MRI. In the context of DWI, there are a few reports applying AI to reduce the noise from the EPI-DWI9,10 or to improve the spatial resolution of the ADC map11. However, there are very few studies applying AI to TSE-DWI.
In this study, a dual step AI strategy is used to remove the noise and undersampling artifacts from the image and to subsequently improve the sharpness of the image. It is hypothesized that the image quality of prostate TSE-DWI and ADC map can be improved significantly by using the proposed AI-based reconstruction technique. The purpose of this study was to acquire distortion-free, high-resolution, and high-SNR prostate TSE-DWI images using the proposed AI-based reconstruction, and to compare the image quality with conventional Compressed-SENSE (C-SENSE) and SmartSpeed AI reconstructions.
Methods
The study was approved by the local IRB, and written informed consent was obtained from all subjects. A total of 5 volunteers were examined on a 3.0T whole-body clinical system (Ingenia Elition X, Philips Healthcare) using a 32-channel phased-array coil. Multi-slice 2D TSE-DWI sequence was acquired in the axial plane.Images were reconstructed using vendor provided prototype (Philips SmartSpeed Precise Image). This AI-based reconstruction technique consists of a series of convolutional neural networks (CNNs): Adaptive-CS-Net12 allows to reconstruct images acquired with C-SENSE based variable density undersampling patterns. This CNN is applied during coil combination, removing noise and undersampling artifacts from the images in order to obtain good image quality from accelerated acquisitions13. Subsequently, Precise Image Net is an AI-model applied to remove ringing artefacts and to replace the traditional zero-filling strategy to increase the matrix size and therewith the sharpness of the images; these type of networks are known as SuperResolution networks14,15. This network is trained on pairs of low- and high-resolution data with k-space crops to induce ringing.
Data consistency checks are implemented to match the resulting k-space with the measured k-space data. The full reconstruction pipeline generates images with improved SNR and sharpness, higher matrix size and reduced ringing artefacts, and can be applied to all 2D cartesian acquisitions. The noise reduction can be controlled using a parameter to the users’ preference.
The image quality was compared between three acceleration methods: C-SENSE, SmartSpeed AI, and SmartSpeed Precise Image. Following parameters were common to all examinations: FOV=320x320 mm2, slice thickness=4 mm, 20 slices, in-plane acquisition resolution 3.0×3.0 mm2, in-plane reconstruction resolution 0.78×0.78 mm2, b-value=0 and 1000 s/mm2, TR=3500 ms, TE=89 ms, acceleration factor=2.5, number of signals averaged=6, and the scan time was 2min13sec. For one subject, EPI-DWI with b-value of 2000 s/mm2 was additionally acquired.
To quantitatively evaluate the influence of the reconstruction technique on ADC values, the transitional zone (TZ) and peripheral zone (PZ) of the prostate were manually segmented for one subject using 3D Slicer16,17. The segmentations were then overlayed to ADC maps and ADC values were extracted for histogram analysis.
Results and Discussions
Figures 2 shows comparison of C-SENSE, SmartSpeed AI, and SmartSpeed Precise Image for high-resolution TSE-DWI. SmartSpeed AI significantly reduced the noise observed in the C-SENSE, especially at b=1000 s/mm2. Visually, SmartSpeed Precise Image showed improved sharpness in both b=0 and 1000 s/mm2 images as well as ADC map. Geometrical distortion was not recognizable for all images. Figure 3 shows the comparison of ADC maps for another subject. SmartSpeed Precise Image provided ADC maps with improved resolution, which may enable evaluating fine pathophysiological structures. Figure 4 shows the histogram of the ADC values extracted from TZ and PZ. In this subject, the mean ADC values for TZ were 1.05, 1.10, and 1.09 x10-3 s/mm2 for C-SENSE, SmartSpeed AI, and SmartSpeed Precise Image, respectively, and for PZ were 1.42, 1.46, and 1.46 x10-3 mm2/s. The slightly lower ADC for the C-SENSE values might be related to the lower SNR and, hence, induced noise bias. Figure 5 shows comparison of C-SENSE, SmartSpeed AI, and SmartSpeed Precise Image for EPI-DWI. Similarly with TSE, SmartSpeed Precise Image showed reduced noise compared with C-SENSE, and improved sharpness compared with C-SENSE and SmartSpeed AI for EPI.Conclusion
Distortion-free, high-resolution, and high-SNR TSE-DWI images for the prostate were acquired using vendor provided prototype AI-based reconstruction technique. Volunteer data demonstrated improved sharpness of DWI image and ADC map compared with conventional techniques, which resulted in improved overall image quality. Further investigation is warranted to assess the influence of this technique on the ADC values.Acknowledgements
No acknowledgement found.References
1. American College of Radiology. PI-RADS Version 2.1. Prostate Imaging-Reporting Data Syst. Published online 2019:1-69. http://www.acr.org/~/media/ACR/Documents/PDF/QualitySafety/Resources/PIRADS/PIRADS V2.pdf
2. Hambrock T, Somford DM, Huisman HJ, et al. Relationship between Apparent Diffusion Coefficients at 3.0-T MR Imaging and Gleason Grade in Peripheral Zone Prostate Cancer. Radiology. 2011;259(2):453-461. doi:10.1148/radiol.11091409
3. Katahira K, Takahara T, Kwee TC, et al. Ultra-high-b-value diffusion-weighted MR imaging for the detection of prostate cancer: Evaluation in 201 cases with histopathological correlation. Eur Radiol. 2011;21(1):188-196. doi:10.1007/s00330-010-1883-7
4. Krishna S, Lim CS, McInnes MDF, et al. Evaluation of MRI for diagnosis of extraprostatic extension in prostate cancer. J Magn Reson Imaging. 2018;47(1):176-185. doi:10.1002/jmri.25729
5. Esses SJ, Taneja SS, Rosenkrantz AB. Imaging Facilities’ Adherence to PI-RADS v2 Minimum Technical Standards for the Performance of Prostate MRI. Acad Radiol. 2018;25(2):188-195. doi:10.1016/j.acra.2017.08.013
6. Pirasteh A, Johnson B, Dimitrov IE, et al. Turbo Spin-Echo Diffusion-Weighted Imaging in Prostate Magnetic Resonance Imaging of Men With Pelvic Hardware. J Comput Assist Tomogr. 2020;44(4):519-526. doi:10.1097/RCT.0000000000001067
7. Mori N, Mugikura S, Miyashita M, et al. Turbo spin-echo diffusion-weighted imaging compared with single-shot echo-planar diffusion-weighted imaging: Image quality and diagnostic performance when differentiating between ductal carcinoma in situ and invasive ductal carcinoma. Magn Reson Med Sci. 2021;20(1):60-68. doi:10.2463/mrms.mp.2019-0195
8. Tamada T, Ueda Y, Ueno Y, Kojima Y, Kido A, Yamamoto A. Diffusion-weighted imaging in prostate cancer. Magn Reson Mater Physics, Biol Med. 2021;(0123456789). doi:10.1007/s10334-021-00957-6
9. Yoneyama M, Yoshida T, Kwon J, Katsumata Y, Zhang S, Van Cauteren M. SNR enhancement in rapid high b-value prostate single-shot DW-EPI utilizing deep learning constrained Compressed SENSE reconstruction. In: ISMRM2022 #3712. ; 2022. doi:10.2214/AJR.09.3004.4.
10. Ueda T, Ohno Y, Yamamoto K, et al. Deep Learning Reconstruction of Diffusion-weighted MRI Improves Image Quality for Prostatic Imaging. Radiology. 2022;303(2):373-381. doi:10.1148/RADIOL.204097
11. Fan M, Liu Z, Xu M, et al. Generative adversarial network-based super-resolution of diffusion-weighted imaging: Application to tumour radiomics in breast cancer. NMR Biomed. 2020;33(8):1-12. doi:10.1002/nbm.4345
12. Pezzotti N, de Weerdt E, Yousefi S, et al. Adaptive-CS-Net: FastMRI with Adaptive Intelligence. arxiv. 2019;(NeurIPS). http://arxiv.org/abs/1912.12259
13. Peeters H, Chung H, Valvano G, et al. Philips SmartSpeed No compromise and robustness.
14. Li Y, Sixou B, Peyrin F. A Review of the Deep Learning Methods for Medical Images Super Resolution Problems. Irbm. 2021;42(2):120-133. doi:10.1016/j.irbm.2020.08.004
15. Chaudhari AS, Fang Z, Kogan F, et al. Super-resolution musculoskeletal MRI using deep learning. Magn Reson Med. 2018;80(5):2139-2154. doi:10.1002/mrm.27178
16. Kikinis R, Pieper SD, Vosburgh KG. 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support. In: Intraoperative Imaging and Image-Guided Therapy. Springer New York; 2014:277-289. doi:10.1007/978-1-4614-7657-3_19
17. 3D Slicer image computing platform. https://www.slicer.org/
Figures
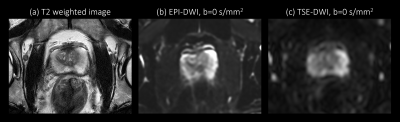
Figure 1. Anatomical T2 weighted image (a) and corresponding EPI-DWI (b) and TSE-DWI (c) images, both acquired with b = 0 s/mm2. This representative EPI-DWI shows higher signal to noise ratio and severe image distortion in the proximity of rectum gas. TSE-DWI is less sensitive to such susceptibility difference, which results in anatomically more accurate delineation of prostate structure.
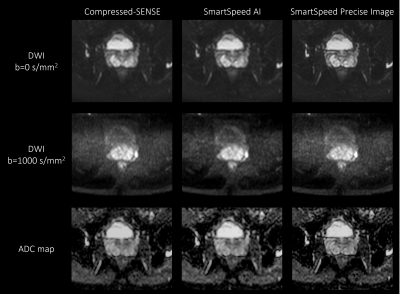
Figure 2. b = 0 (upper row), b = 1000 s/mm2 (middle row) images, and ADC maps (lower row) of the prostate in a healthy volunteer obtained with TSE-DWI, for C-SENSE (left), SmartSpeed AI (middle), and SmartSpeed Precise Image (right) reconstructions.

Figure 3. ADC maps of the prostate in a healthy volunteer obtained with TSE-DWI, for C-SENSE (a), SmartSpeed AI (b), and SmartSpeed Precise Image (c) reconstructions.

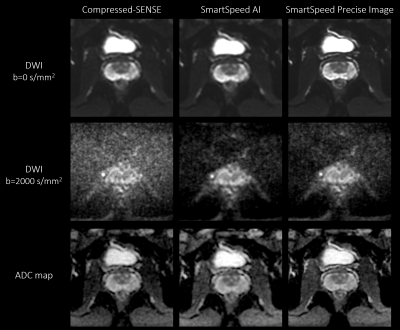
Figure 5. b = 0 (upper row), b = 2000 s/mm2 (middle row) images, and ADC maps (lower row) of the prostate in a healthy volunteer obtained with EPI-DWI, for C-SENSE (left), SmartSpeed AI (middle), and SmartSpeed Precise Image (right).