4721
23Na MRI at 21.1T reveals the Impact of Estrogen Deprivation in Preclinical Migraine
Dayna L. Richter1,2, Samuel Holder1,2, and Samuel Colles Grant1,2
1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States
1National High Magnetic Field Laboratory, Florida State University, Tallahassee, FL, United States, 2Chemical & Biomedical Engineering, FAMU-FSU College of Engineering, Tallahassee, FL, United States
Synopsis
Keywords: Non-Proton, Neuro, Migraine, Extreme field
Migraine disproportionately affects women in part due to menstrual migraine, linked to 17β-estradiol deprivation. This study implements 23Na MRI at 21.1 T in female Sprague-Dawley rats to evaluate the impact of estradiol deprivation on widespread sodium increases previously reported in the male model. Ovariectomy was implemented, and animals dosed with controlled estradiol to mimic the natural estrus cycle. It was found that females at physiological estradiol concentrations are resilient to sodium increases in the brainstem and CSF, while estradiol deprivation removes this resiliency. This interaction may play a role in menstrual migraine, and the clinical migraine gap between sexes.Introduction
Majority of migraineurs are women, but most preclinical work is done with male animal models due to fluctuations of reproductive hormones. Previously in the male Sprague-Dawley rat model, widespread increases in 23Na signal were observed under the nitroglycerin (NTG) chemical model of migraine1. These increases also are observed in the cerebrospinal fluid (CSF) of human migraineurs2. It has been proposed that increased neuronal excitement from these Na+ increases may play a role in the initial onset of migraine, but it is unclear how sex hormones may impact these Na+ increases. Clinically, women report increased migraine frequency and severity at the beginning of their menstrual cycle, when 17β-estradiol (E2) concentrations are lowest3. In this work, 3D 23Na MRI at 21.1 T was used to investigate the impact of estradiol deprivation on NTG-based central sensitization in the female Sprague-Dawley model.Methods
Animal Methods: To control estrogen levels, female Sprague-Dawley rats underwent ovariectomies 8 d prior to scanning. Rats were randomly separated into two groups: a depleted E2 group (0-μg E2, n = 4), and physiological E2 group (2-μg E2, n = 4 ). Groups were administered their E2 dose in a sesame oil vehicle on the fourth and eighth day post-operation to mimic the natural rat estrous cycle. Prior to scanning, animals were anesthetized with 5% isoflurane for implantation of an intraperitoneal (IP) line that enabled central sensitization onset in situ via administration of 10-mg/kg-body weight nitroglycerin (NTG). Animals were maintained at 2-3% isoflurane during scanning.MRI Methods: 3D 23Na Chemical Shift Imaging (CSI) (Fig. 1) was acquired using the 21.1-T, 900-MHz vertical scanner at the National High Magnetic Field Laboratory in Tallahassee, FL. Acquired resolution was 1x1x3 mm. Three baseline scans were acquired before NTG injection; scans were acquired every 20 min out to 2 h post-injection. Reconstruction and segmentation were performed in MATLAB 2020b with segmentation of the following regions of interest: third ventricle, thalamus, cisterna magna and brainstem. Mixed model analysis with post-hoc Tukey HSD tests were performed in JMP Pro 15, with p<0.05 used to identify all significances.
Results and Discussion
As anticipated, removal of E2 from the female Sprague-Dawley rat model resulted in sodium changes inline with those previously found in males. Within the brainstem, a structure critical to the onset of migraine-induced nociception due to its association with the trigeminovascular system, the physiological dosed (2-μg E2) group remained resilient to any sodium increases. However, the brainstem of the E2 deprived (0-μg E2) group saw increases significant to both baseline and the physiological group (Fig. 2). Likewise, CSF regions showed significant increases in 23Na signal with E2 deprivation but not with physiological E2 supplementation. With E2 deprivation, the cisterna magna surrounding the brainstem (Fig. 3) remained progressively elevated throughout the full time course following NTG injection while the 3rd ventricle (Fig. 4) reached and maintained significance first at 50 min post NTG. While it was anticipated the thalamus would see some impact from E2 deprivation, as it is a central relay for nociceptive and sensory information, no statistical significances were observed (Fig. 5) for either 0- or 2-μg E2.With the complete removal of circulating E2, the female 23Na signals in the brainstem and CSF display the same trends as those previously reported for male rats1, though the absolute magnitude of this precent sodium change is less for females. With supplementation of E2 to a controlled average physiological level without estrous fluctuations, sodium increases were largely suppressed, which reflects sodium MRI findings in free cycling female rats (data not shown). As such, the female sodium data mirror the current clinical understanding of menstrual migraine, with E2 deprivation resulting in sensitizing increases in neural sodium.
Conclusion
23Na CSI was implemented at 21.1 T to examine the impact of estrogen deprivation on preclinical migraine within the female rat model. Animals ovariectomized then dosed with physiological estradiol concentrations were resilient to NTG-based increases in neural sodium, while estradiol-deprived animals were susceptible. This interaction between estradiol and neural sodium may help explain the onset of menstrual migraine in humans.Acknowledgements
All work has been done in accordance with the Huntington Medical Research Institute and Florida State University Animal Care and Use Committees. This work was supported by the US NIH (R01-NS072497) and the US National High Magnetic Field Laboratory, which is supported by the National Science Foundation (DMR-1644779) and the State of Florida.References
1. Abad N, Rosenberg JT, Hike DC, Harrington MG, Grant SC. Dynamic Sodium Imaging at Ultra-High Field Reveals Progression in a Preclinical Migraine Model. Pain. 2018;159(10):2058-2065. doi:10.1097/j.pain.0000000000001307
2. Harrington MG, Fonteh AN, Cowan RP, et al. Cerebrospinal Fluid Sodium Increases in Migraine. Headache J Head Face Pain. 2006;46(7):1128-1135. doi:10.1111/j.1526-4610.2006.00445.x
3. Mannix LK, Calhoun AH. Menstrual migraine. Curr Treat Options Neurol. 2004;6(6):489-498. doi:10.1007/s11940-004-0006-7
Figures
An example image of the 3D 23Na CSI.
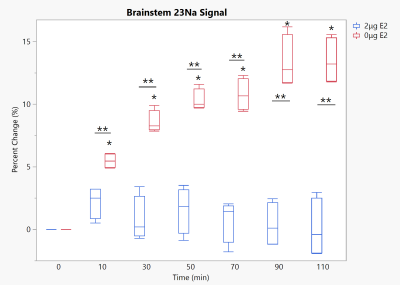
Brainstem 23Na signal under 10 mg/kg NTG. * - significant to baseline, ** - significance between groups. p<0.05 for all significances. Timepoint 0 reflects baseline scans prior to NTG injection.
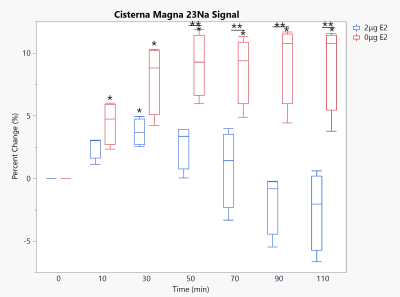
Cisterna Magna 23Na signal under 10 mg/kg NTG. * - significant to baseline, ** - significance between groups. p<0.05 for all significances. Timepoint 0 reflects baseline scans prior to NTG injection.
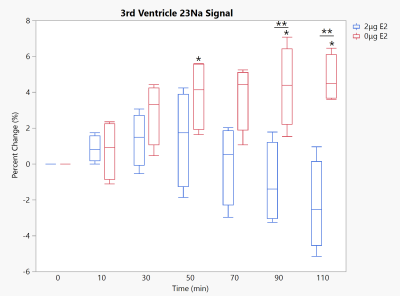
Third Ventricle 23Na signal under 10 mg/kg NTG. * - significant to baseline, ** - significance between groups. p<0.05 for all significances. Timepoint 0 reflects baseline scans prior to NTG injection.
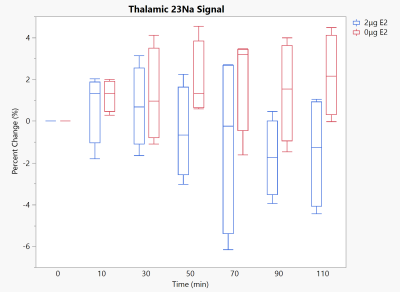
Thalamic 23Na signal under 10 mg/kg NTG. p<0.05 for all significances, none are observed. Timepoint 0 reflects baseline scans prior to NTG injection.
DOI: https://doi.org/10.58530/2023/4721