4719
7-T 39K/23Na MRI for assessment of ionic balance in patients exhibiting hypokalemic periodic paralysis1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Department of Radiology and Biomedical Imaging, Magnetic Resonance Research Center, Yale University, New Haven, CT, United States, 3Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Department of Neurology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 5Centre for Rare Diseases Erlangen (ZSEER), University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 6Institute of Diagnostic and Interventional Radiology, Pediatric Radiology and Neuroradiology, University Medical Center Rostock, Rostock, Germany
Synopsis
Keywords: Non-Proton, Muscle, Potassium
Combined 39K/23Na MRI at 7T has become feasible recently after technical advances of 39K MRI. Here we assessed ion homeostasis alterations, especially tissue potassium and sodium concentrations (TPC/TSC) in a small pilot cohort of patients of hypokalemic periodic paralysis (HypoPP). Depending on the severity of fatty involvement of muscles, TSC was elevated in slightly to moderately involved muscles and TPC showed a tendency to decrease in an inverse correlated fashion. Almost entirely fatty infiltrated muscle showed a decrease of TSC and TPC. Therefore, combined 39K/23Na MRI could help to examine the pathophysiological processes in HypoPP and other muscular channelopathies.Introduction
Ion channel mutations lead to a variety of seldom inherited muscular diseases (channelopathies). This disease cohort includes the hypokalemic periodic paralysis (HypoPP), where non-selective cation leaks can lead to paralytic attacks and, in later stages, to permanent muscle weakness1. Most common are mutations of the calcium channel protein CACNA1S1. Knowledge of ion homeostasis of HypoPP is crucial for improved understanding of pathophysiology and potentially also treatment monitoring.So far,sodium-23-(23Na)- and chlorine-35-(35Cl)-imaging have been used to assess ion homeostatsis in HypoPP2,3. Recent advances have made it feasible to perform potassium-39-(39K)-MRI in vivo with sufficient signal-to-noise ratio, however studies within patients with muscular diseases are not yet available. Therefore, we aimed to perform 39K-MRI measurements of the lower leg combined with 23Na-MRI to study ion homeostasis of HypoPP-patients.
Methods
Three unrelated patients with genetically confirmed HypoPP (mutation CACNA1S-R528H; 2f, 1m, mean age 54.3±9.6) and three healthy controls (1f, 2m, mean age 49.7±17.6) were prospectively included in our study. As coldness is known as a provocation factor for episodic paralysis, the examined lower leg was cooled for 20 minutes with ice-water bags1,2. 1H-MRI was performed solely at rest, 23Na- and 39K-MRI also after cooling. The study was approved by the local review board.1H-MRI of the left lower leg was performed at a 3T whole-body system (Magnetom Vida, Siemens Healthcare GmbH, Erlangen, Germany) using an 18-channel flexible RF-coil and included a T1-weighted turbo-spin echo sequence to detect potential lipomatous and a T2-weighted TI-IR-(STIR)-sequence to detect edematous alterations. 23Na- and 39K-MRI data were acquired at a 7T whole-body system (Magnetom Terra, Siemens Healthcare GmbH) using a dual-tuned, quadrature birdcage 23Na/39K-RF-coil tailored for examinations of the lower leg. An acquisition-weighted Stack-of-Stars (AW-SOSt) sequence4 (Table 1). A 23Na-inversion-revovery (23Na-IR) sequence served to reduce 23Na-signal from liquid sodium and to result in a stronger weighting towards intracellular Na2.
Intramuscular fat and edema assessed on 1H-images were each graded using a four-point semi-quantitative scale (1 := healthy tissue; 4:= complete fatty infiltration/homogeneous edema3).
For quantification of 23Na/39K-data, 1H-T1-TSE images were co-registered using a non-rigid registration-tool5 on 23Na-datasets and segmented semi-automatically using Dafne6 into individual muscles, the entire muscle tissue and subcutaneous adipose tissue4 (Fig. 1). Blood vessels were extracted using a threshold within 23Na-MRI images. 23Na- and 39K-signal intensities were corrected for partial-volume effects7 and relaxation effects8. TSC- and TPC-values were calculated based on five external references containing NaCl- and KCl-solutions ([Na+]/[K+] = 10/240,20/210,25/180,30/150,40/120 mM).
Results
Fatty muscle infiltration was present in all HypoPP-patients, but showed a wide pattern of disease progression from grade 4 (complete fatty infiltration) down to only slight or no changes (grade 1-2) within patients and between different muscle compartments. The gastrocnemicus medialis (GM) muscle was affected most severely, the extensor muscles mostly spared. Muscle edema were present in all patients and ranged from slight to marked changes, most prominent in the flexor lodge. All patients exhibited normal muscle strength at rest.Figures 2 and 3 show MR-images in two HypoPP-patients with different disease progression. The most severely affected GM muscle shows a concomitant decrease of TSC and TPC when complete fatty infiltration is present (Fig. 2), whereas visually more intact GM muscles of the two other patients (where no or slight fatty infiltration is present) show a tending to an increase of TSC and a less severe decrease of TPC (Fig. 3), compared to healthy controls (Fig. 4) (Na+]/[K+] = 14.1/9.4, 30.1/93.2, 43.8/90.9; mean of controls = 26.9±7.4/104.4±4.5mM). Soleus (SOL) muscle exhibited quite similar effects, here the TSC of partly fatty infiltrated SOL of most severe effected patient matches the one of healthy controls (Na+]/[K+] = 26.2/12.9, 34.7/85.7, 32.9/88.2; mean of controls = 26.7±5.7/105.0±6.3 mM). On 1H-MRI almost intact tibialis anterior (TA) muscle exhibit an increase of TSC and a decrease of TPC in all patients (Na+]/[K+] = 35.2/72.6, 26.6/77.5, 36.7/101.8; mean of controls = 24.6±6.7/104.8±11.1 mM).23Na-IR-signal showed the same tendencies as TSC. Overall, 23Na-IR was the only contrast exhibiting changes after cooling within two patients (Fig. 2), namely a tendency to an increase of signal intensity. One of them presented a concomitant slight muscle weakness of foot/toe-extension after cooling (MRC-grade 4-5), other patients showed normal strength.
Discussion
In accordance with previous studies we observed an increase of TSC in muscles of HypoPP-patients2,3. As soon as fatty infiltration takes over in later disease stages, the effect of an increase of TSC in more intact muscle regions is outweighed by the effect of a decrease of TSC in fatty muscle parts, when calculating TSC partial-volume corrected. TPC show a tendency to a decrease in HypoPP-patients, inversely-correlated to TSC. TSC/TPC-changes apply in part also for almost 1H-MRI-intact muscles. No significant changes of TSC and TPC could be observed after cooling. Only 23Na-IR signal, weighted more towards intracellular Na, showed a slight tendency to a signal increase in HypoPP-patients with however high variations, similar to a previous study2.Conclusion
Our results indicate that 39K-MRI has the potential to show alterations of K-ion balance in patients with muscular channelopathies, also in almost 1H-MRI-intact muscles, here shown for a pilot cohort of HypoPP-patients and could form in combination with 23Na an important building block for a further understanding of these rare diseases.Acknowledgements
We are grateful to our patients for their participation. We thank the Imaging Science Institute (Erlangen, Germany) for providing us with measurement time at the 3T MRI system.References
(1) Jurkat-Rott, K.; Weber, M.-A.; Fauler, M.; Guo, X.-H.; Holzherr, B. D.; Paczulla, A.; Nordsborg, N.; Joechle, W.; Lehmann-Horn, F. K+-dependent paradoxical membrane depolarization and Na+ overload, major and reversible contributors to weakness by ion channel leaks. Proceedings of the National Academy of Sciences of the United States of America 2009, 106 (10), 4036–4041.
(2) Nagel, A. M.; Amarteifio, E.; Lehmann-Horn, F.; Jurkat-Rott, K.; Semmler, W.; Schad, L. R.; Weber, M.-A. 3 Tesla sodium inversion recovery magnetic resonance imaging allows for improved visualization of intracellular sodium content changes in muscular channelopathies. Investigative Radiology 2011, 46 (12), 759–766.
(3) Weber, M.-A.; Nagel, A. M.; Marschar, A. M.; Glemser, P.; Jurkat-Rott, K.; Wolf, M. B.; Ladd, M. E.; Schlemmer, H.-P.; Kauczor, H.-U.; Lehmann-Horn, F. 7-T (35)Cl and (23)Na MR Imaging for Detection of Mutation-dependent Alterations in Muscular Edema and Fat Fraction with Sodium and Chloride Concentrations in Muscular Periodic Paralyses. Radiology 2016, 281 (1), 326.
(4) Gast, L. V.; Baier, L.-M.; Chaudry, O.; Meixner, C. R.; Müller, M.; Engelke, K.; Uder, M.; Heiss, R.; Nagel, A. M. Assessing muscle-specific potassium concentrations in human lower leg using potassium magnetic resonance imaging. NMR in Biomedicine 2022, e4819.
(5) Klein, S.; Staring, M.; Murphy, K.; Viergever, M. A.; Pluim, J. P. W. elastix: a toolbox for intensity-based medical image registration. IEEE Trans. Med. Imaging 2010, 29 (1), 196–205.
(6) Santini, F.; Wasserthal, J.; Agosti, A. Deep Anatomical Federated Network (Dafne). https://dafne.network. Accessed November 9, 2022.
(7) Niesporek, S. C.; Hoffmann, S. H.; Berger, M. C.; Benkhedah, N.; Kujawa, A.; Bachert, P.; Nagel, A. M. Partial volume correction for in vivo (23)Na-MRI data of the human brain. NeuroImage 2015, 112, 353–363.
(8) Gast, L. V.; Völker, S.; Utzschneider, M.; Linz, P.; Wilferth, T.; Müller, M.; Kopp, C.; Hensel, B.; Uder, M.; Nagel, A. M. Combined imaging of potassium and sodium in human skeletal muscle tissue at 7 T. Magnetic Resonance in Medicine 2021, 85 (1), 239–253.
Figures
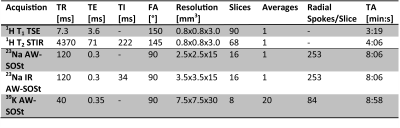
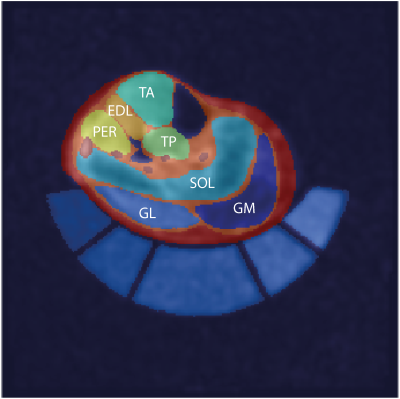
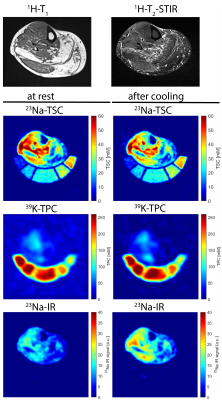
Figure 2: Axial 1H-MR, 23Na- and 39K-MR images of the lower leg of a HypoPP patient at a late disease stage. 1H-MR data were acquired solely at rest, 23Na- and 39K-MR at rest and after cooling. TSC and TPC images display relaxation-corrected data. 23Na-IR data are indirectly calibrated with correction factors obtained from 23Na-TSC data. Fatty infiltrated GM muscle shows decreased TSC and TPC, more intact GL and TA muscle increase of TSC and decrease of TPC. 23Na-IR displays an increase after cooling.
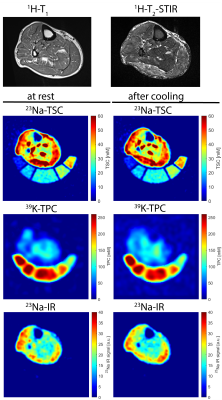
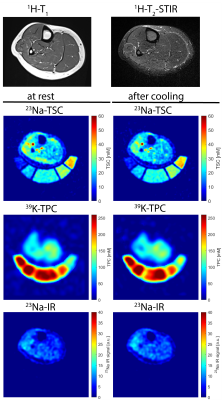
Figure 4: Axial 1H-MR, 23Na- and 39K-MR images of the lower leg of a healthy control. 1H-MR data were acquired solely at rest, 23Na- and 39K-MR at rest and after cooling. TSC and TPC images display relaxation-corrected data. 23Na-IR data are indirectly calibrated with correction factors obtained from 23Na-TSC data. Low TSC and high TPC values, only slightly increased TSC values within extensor digitorum longus (EDL) and peroneus (PER) muscles, partly suppressed by 23Na-IR.