4711
Entropy as a Novel Predictor of Cardiovascular Events in Patients with Left Ventricular Noncompaction1Department of Radiology, Shandong Provincial Hospital, Shandong University, Jinan, China, 2Siemens Healthineers, Shanghai, China, 3College of Medical Imaging, Shanghai University of Medicine & Health Science, Shanghai, China, 4Department of Radiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
Synopsis
Keywords: Myocardium, Heart
The prognosis of LVNC is remarkably heterogeneous, with heart failure (HF), ventricular arrhythmias (VAs) and systemic embolisms (SEs) being the most frequent cardiovascular complications but no specific recommendations are available at present. In the present study, we aimed to investigate whether LV entropy could efficiently predict major adverse cardiac events (MACEs) in patients with LVNC incremental to established clinical and imaging risk markers.Introduction
The prognosis of LVNC is remarkably heterogeneous, with heart failure (HF), ventricular arrhythmias (VAs) and systemic embolisms (SEs), and the risk stratification of left ventricular noncompaction (LVNC) is still ambiguous. Myocardial fibrosis detected by CMR using LGE imaging is the current gold standard to noninvasively visualize the underlying scar architecture and inform towards risk of scar-related cardiac events (1,2). However, this signal intensity (SI)-based method relies on operator-defined areas with categorical thresholds or areas with remote myocardium as reference to assess the extent of pixels exceeding the setting threshold (3), and mainly focus on measuring scar presence, pattern, and extent, which have limitations in assessing non-enhanced regions of the myocardium (4). LV entropy derived from late gadolinium enhancement (LGE) in cardiac magnetic resonance (CMR) may serve as the substrate of major adverse cardiovascular events (MACEs). The purpose of this study was to investigate the value of LV entropy, as a novel measurement of myocardial heterogeneity, for predicting MACEs in LVNC patients.Methods
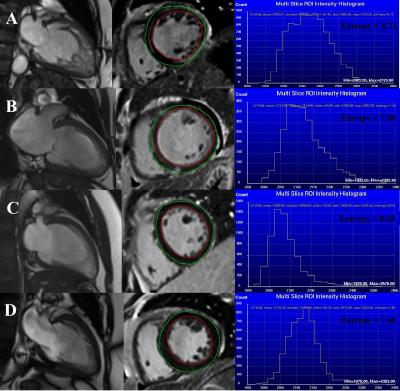
143 consecutive patients undergoing CMR for LVNC were included and followed for MACEs defined by all-cause death, ventricular arrhythmia (VA) requiring therapy, systemic embolisms or heart failure hospitalization. All CMR examinations were performed using 3T scanners (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany). The conventional analysis was performed offline using dedicated commercially available software (Medis Suite v3.1, Medis, Leiden, the Netherlands) following standardized recommendations by two experienced researchers blinded to clinical data. For the LV entropy calculations, epicardial and endocardial contours were manually delineated on the short-axis images of LGE with careful exclusion of any blood pool signal. LV entropy values were directly derived from the distribution of pixel signal intensities of the LV myocardium on LGE images and automatically generated using Research Mass (Leiden University Medical Center, Leiden, the Netherlands) according to the following formula (3,5):Entropy = - Σni=1P(xi)logbP(xi)
where P(xi) is the probability distribution of signal intensities, x is signal intensity, and b is any chosen base (Research Mass uses 2). The comparisons among clinical variables, CMR-based parameters and entropy were performed with χ2 tests, Student's t-tests, Mann-Whitney U tests for categorical variables, normally, and skewed distributed continuous variables, respectively. A p-value < 0.05 were considered to be statistically significant.
Results
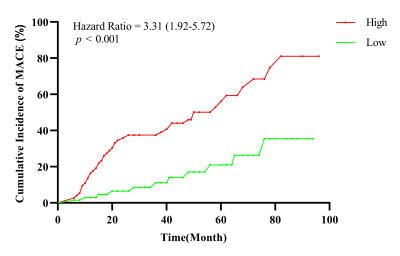
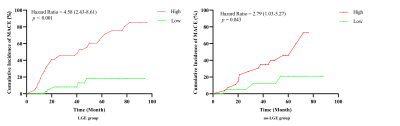
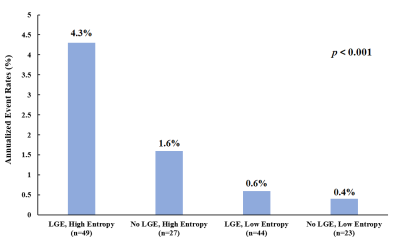
One hundred and forty-three patients (mean age 40 years, 64.3% male) were followed for a median of 3.2 years and fifty-two (36.4%) experienced MACE. Left ventricular end-diastolic diameter (LVEDD), LV end-diastolic volume (LVEDV) index, LV end-systolic volume (LVESV) index, LV ejection fraction (LVEF), LGE extent and LV entropy showed association with MACE. LV entropy maintained independent association with MACEs (HR: 2.49; 95% CI: 1.22-5.10; p < 0.001) in multivariable analysis. Entropy was also strong independent predictor of MACEs in patients with and without LGE (HR: 2.49, 95% CI 1.97-3.73, p < 0.001; HR: 1.85, 95% CI: 1.23-3.95, p = 0.048, respectively).Discussion
LV entropy assessment in patients with myocardial scar indirectly includes the features of the peri-fibrosis area. Heterogeneity in this area has been proven to represent myocardial tissue that is arrhythmogenic (6). LV entropy derived from the entirety of the signal intensity distribution is threshold-independent and possibly allows detection of more gradual differences in myocardial texture (7).Conclusions
LV entropy can predict MACEs in LVNC patients and provided incremental prognostic value on top of LVEF and LGE. LV entropy may help risk stratification in LVNC patients with absence of myocardial scar.Acknowledgements
NoneReferences
1. Hennig A, Salel M, Sacher F et al. High-resolution three-dimensional late gadolinium-enhanced cardiac magnetic resonance imaging to identify the underlying substrate of ventricular arrhythmia. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology 2018;20:f179-f191.
2. Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet (London, England) 2001;357:21-8.
3. Androulakis AFA, Zeppenfeld K, Paiman EHM et al. Entropy as a Novel Measure of Myocardial Tissue Heterogeneity for Prediction of Ventricular Arrhythmias and Mortality in Post-Infarct Patients. JACC Clin Electrophysiol 2019;5:480-489.
4. Di Marco A, Anguera I, Schmitt M et al. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart failure 2017;5:28-38.
5. Muthalaly RG, Kwong RY, John RM et al. Left Ventricular Entropy Is a Novel Predictor of Arrhythmic Events in Patients With Dilated Cardiomyopathy Receiving Defibrillators for Primary Prevention. JACC Cardiovascular imaging 2019;12:1177-1184.
6. Schmidt A, Azevedo CF, Cheng A et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation 2007;115:2006-14.
7.Antiochos P, Ge Y, van der Geest RJ et al. Entropy as a Measure of Myocardial Tissue Heterogeneity in Patients With Ventricular Arrhythmias. JACC Cardiovascular imaging 2022;15:783-792.
Figures