4710
Cardiovascular magnetic resonance findings in Danon disease:A case series of a big family
Xiaolong Liu1, Zhanguo Sun1, Ning Zhai1, and Xiuzheng Yue2
1Department of Radiology, Affiliated Hospital of Jining Medical University, Jining, China, 2Philips Healthcare (Beijing), Beijing, China
1Department of Radiology, Affiliated Hospital of Jining Medical University, Jining, China, 2Philips Healthcare (Beijing), Beijing, China
Synopsis
Keywords: Heart, Myocardium
Heart involvement is the leading cause of death of Danon disease (DD). However, the cardiac magnetic resonance (CMR) features of DD are not well understood. Hence, we present seven DD patients with from the same family with follow-up since 2017. In our cohort, the typically CMR features of DD were left ventricle (LV) hypertrophy, late gadolinium enhancement of LV, diminished strain, and preserved or reduced LV ejection fraction. Late gadolinium enhancement most commonly involves the LV free wall and the right ventricle insertion points. Moreover, these CMR changes in male patients appeared earlier and more significant, and progressed faster.Introduction
Danon disease (DD) is an extremely rare X-linked dominant lysosomal glycogen storage disease. Heart involvement is the leading cause of death in patients with DD1. Cardiac magnetic resonance (CMR) is helpful to evaluate the cardiac structure, function, and tissue characteristics in patient with DD2. However, due to the rarity of DD worldwide, these previous studies have mainly concentrated on limited patient numbers with different mutation types from different families2,3. There is still a lack of detailed information about the CMR features and the follow-up changes in large cohorts of patients. In this study, we present seven patients with DD from the same family with long term follow-up to elucidate the main features of it, specially focusing on its CMR findings and the evolutions.Methods
Date acquisitionThe study was approved by the IRB and signed inform consent was obtained from all study participants. Seven patients with DD were enrolled in this study. CMR were performed on 3.0 T MRI scanners (Ingenia, Philips Healthcare, Best, The Netherlands) with 32 channel cardiac coil. The cardiac short-axis orientation, two-, and four-chamber view images were acquired using balanced steady-state free precession cines. Late gadolinium enhancement (LGE) imaging was acquired using phase-sensitive inversion recovery reconstruction with a standard fast low-angle shot inversion recovery sequence 10~15 min after the administration of 0.2 mmol/kg gadolinium-DTPA (Magnevist, Bayer Healthcare, Berlin, Germany).Date analysisDate was transferred to an offline workstation with the commercially available software (CVI42, Circle Cardiovascular Imaging, Calgary, Canada) for analysis. Left ventricle end-systolic volume, LV end-diastolic volume, LV ejection fraction (LVEF), cardiac output and LV mass were measured and indexed to body surface area. Strains and strain rates of LV were derived from cine images in two-chamber, four-chamber views, and short-axis orientation using CMR feature-tracking. Late gadolinium enhancement was interpreted by two experienced CMR readers and recorded on a 17-myocardial-segment model of the LV. Late gadolinium enhancement was semi-automatically quantified by setting a signal intensity threshold at 5 SD above the mean intensity of a reference region with no visual evidence of LGE.Result
By summarizing the genetic analysis and medical history, the pedigree of the family was obtained (Figure 1). And the CMR features of seven patients (Ⅲ-2, Ⅲ-3, IV-1, IV-2, IV-4, IV-5, and IV-6) are summarized in Table 1. Three young female patients presented with normal cardiac morphology. Four patients (two young male and two middle-aged female) presented with LV hypertrophy, and mostly with septal thickening (3/4). Except for one case with decreased LVEF, the LVEF in the other six cases were normal. The strain data of was diminished in varying degrees among the four adult patients and was normal among the three teenagers. Five patients presented with LGE (Figure 2), and the proportion ranged from 36% to 74.3% (median value 48.45%). The most frequent location of LGE was LV free wall (5/5), followed by right ventricle (RV) insertion points (4/5) and intraventricular septum (IVS) (2/5). Mild to moderate hyperintensity on T2-weighted images were revealed in all of the five patients with LGE and overlapped with the LGE areas. Five patients showed resting perfusion defect. All areas of perfusion defect occurred in areas of LGE. Follow-up for all the seven patients lasted to August 2022. None of the five female patients had significant worsening of symptoms. However, the two young male patients showed marked worsening of their cardiac symptoms and CMR data (summarized in Table 1). The thick of LV wall was decreased, while the thickness of RV free wall was increased gradually. The LVEF and strain decreased year by year. The extent of LGE was increased with the involvement of the IVS and RV free wall (Figure 3). In addition, significant low T2 signal foci appeared within the LGE areas.Discussion and conclusions
The typically CMR features of DD were LV hypertrophy, LGE of LV, diminished strain, and preserved or reduced LVEF. Moreover, hyperintensity on T2-weighted images and resting perfusion defect were not uncommon in patients with DD. Consistent with the clinical characteristics, these CMR changes in male patients appeared earlier and more significant, and progressed faster than female patients. The areas of LGE corresponded closely in distribution and extent to the fibrosis and scarring of DD. LGE most commonly involve the LV free wall and the RV insertion points, rather than the IVS. Furthermore, most the LGE areas exhibit transmural or intramyocardial distributions. The patterns of LGE in DD are unlike those of typical hypertrophy cardiomyopathy, amyloidosis, and sarcoidosis and help in the different diagnosis. Strain alterations have the potential and prior value in detecting cardiac dysfunction even with normal LVEF. Both low T2 signal and defected resting perfusion appeared at the end stage and may be used to reflect the extent of fibrosis. Therefore, CMR is of great value in the diagnosis and follow-up of patients with DD.Acknowledgements
No acknowledgement found.References
1.Rowin EJ, Maron BJ, Arkun K, Maron MS. Anatomic validation of late gadolinium enhancement as evidence of myocardial scarring in LAMP2 cardiomyopathy. Eur Heart J 2017;38(31):2444.2.Wei X, Zhao L, Xie J, et al. Cardiac Phenotype Characterization at MRI in Patients with Danon Disease: A Retrospective Multicenter Case Series. Radiology 2021;299(2):303-310.
3. Piotrowska-Kownacka D, Kownacki L, Kuch M, et al. Cardiovascular magnetic resonance findings in a case of Danon disease. J Cardiovasc Magn Reson 2009;11(1):12.
Figures
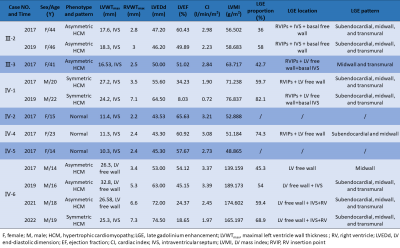
Table 1. Cardiac structure, function, and late gadolinium enhancement characteristics of Danon disease on CMR.
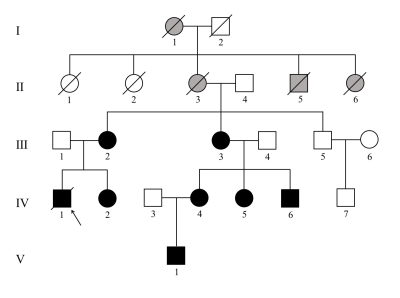
Figure 1. Pedigree of the family with Danon disease. Circles, female individuals; squares, male individuals; slashes symbols, deceased individuals; black shapes, affected subjects; grey shapes, suspected subjects; white shapes, unaffected subjects; arrow, proband.
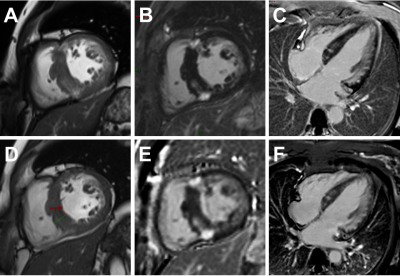
Figure 2. A 49-year-oldfe male patient (Ⅲ-2) with Danon disease. Cine cardiac image in short-axis view showed increased thickness of intraventricular septum (A, D). Late gadolinium enhancement involving anterior, lateral walls, both right ventricle insertion points, and intraventricular septum. The extent of late gadolinium enhancement in 2019 (E, F) showed mild to moderate progression compared to 2017 (B, C).
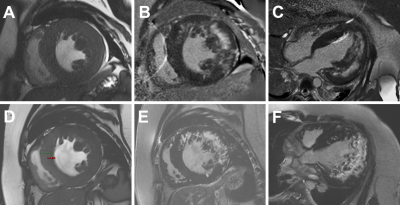
Figure 3. A 19-year-old male patient (IV-6) with Danon disease. Cine cardiac image showed short-axis view show symmetrically increased thickness of left ventricle wall (A, D). Late gadolinium enhancement involving anterior, lateral walls. A small LGE region was present in the anterior intraventricular septum. The extent of late gadolinium enhancement in 2021(E, F) showed significant progression compared to 2019 (B, C).
DOI: https://doi.org/10.58530/2023/4710