4708
Cardiac PET-MRI to measure cardiac efficiency access Alginate intramyocardial injections in a porcine heart failure model1DelBeat, Berkeley, CA, United States, 2University of California San Francisco, San Francisco, CA, United States, 3University of California, San Francisco, San Francisco, CA, United States, 4Lawrence Berkeley National Laboratory, Berkeley, CA, United States, 5Cleveland Clinic, Cleveland, OH, United States
Synopsis
Keywords: Cardiomyopathy, Heart, Hear efficiency
Nearly 70% of heart attacks (myocardial infarctions, MI) cases lead to a condition known as heart failure (HF), where the left ventricle (LV) remodels. Previous alginate and stem cell intramyocardial injection studies reported mixed outcomes using the LV global volumetric function and local myocardium mechanical response (strains and stresses)1,2. Such measures involve unvalidated assumptions yielding erroneous results3. We conducted a swine HF study treated with a particular alginate intramyocardial injection; however, instead of using the mechanical response, we directly measured the metabolic energy consumed by the myocardium before and after treatment using Positron emission tomography–magnetic resonance images (PET-MRI)4.Introduction
Nearly 70% of heart attack (myocardial infarctions, MI) cases lead to a condition known as heart failure (HF), where the left ventricle (LV) remodels. Previous alginate and stem cell intramyocardial injection studies reported mixed outcomes using the LV global volumetric function and local myocardium mechanical response (strains and stresses)1,2. Such measures involve unvalidated assumptions yielding erroneous results3. We conducted a swine HF study treated with a particular alginate intramyocardial injection; however, instead of using the mechanical response, we directly measured the metabolic energy consumed by the myocardium before and after treatment using Positron emission tomography–magnetic resonance images (PET-MRI)4.Method
Animal Model: A porcine model of ischemic cardiomyopathy was used to investigate biomechanical cardiac efficiency. 8 weeks post-MI, verification of a significant decrease in ejection fraction (EF) (below 35%) and an increase in LVEDD of at least 25% were verified to indicate the development of cardiomyopathy. Subjects underwent post-MI PET/MRI imaging followed by endocardial injections of either alginate or PBS. Repeat imaging was performed 8 weeks after injections. PET-MRI: The metabolism in the heart is closely associated with oxidative phosphorylation; the oxidation of 11C-acetate is an excellent probe for estimating MVO2 by measuring the rate of efflux of 11C-CO2. We measured the regional metabolic energy consumption (MVO2) noninvasively using 30-min dynamic 11C-acetate PET. Along with the 30-min PET acquisition, CINE, tagged cardiac MR, and 3D fast spoiled gradient-echo T1-weighted imaging (i.e., LAVA-flex on the GE platform) were acquired. After correcting PET-derived blood activity for the 11C-CO2 contribution, blood and myocardial TACs were used with a 2-tissue compartment model to estimate the wash-in (K1) and wash-out (k2) rates. The estimated k2-values were converted to energy using the equation MVO2=135(k2)-0.96 ml/100gm/min by assuming 1 ml oxygen = 21 joules. Analysis: We measured the LV cavity volume and pressure with an intracardiac pressure transcutaneous catheter (MicroCath, Millar Instruments). We segmented the cardiac CINE MR images at every time frame and computed the LV-cavity volumes and the ejection fractions (EF). The LV-pressure drop was calculated using Delbeat Finite-Element-based (FE) computational fluid dynamics tool and measured 2D-phase contrast MR image5,6. The overall LV efficiency was computed using the work done by the LV wall on the displaced blood divided by the total metabolic energy measured by PET (MVO2). We are calculating the local tissue strain energy of the LV wall, which will enable us to compute local tissue mechanical efficiencies. We used diffusion tensor imaging (DTI)7 to compare the myofiber morphology of all treated and controlled subjects.Results
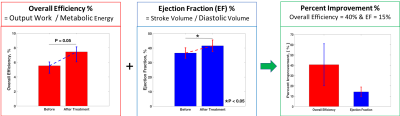
Analysis of the PET-MRI cases showed that the overall efficiency (borderline significant) and EF (P < 0.05) improved upon endocardial alginate injection (Figure-1). The treated LV-cavity sizes were reduced.Discussion
The alginate injection improvement rate varied because of MI sizes, injection sites, numbers, and amounts. However, the outcome suggests a positive outcome and necessitates a predictive tool to estimate optimal injection parameters. Moreover, the efficiency calculation embeds the myocardial strains, stresses, and blood flow losses; hence, overall efficiency is more reliable for assessing cardiac function than EF and other cardiac measures.Acknowledgements
No acknowledgement found.References
1. Choy JS, Leng S, Acevedo-Bolton G, Shaul S, Fu L, Guo X, Zhong L, Guccione JM, Kassab GS. Efficacy of intramyocardial injection of Algisyl-LVR for the treatment of ischemic heart failure in swine. Int J Cardiol. 2018.
2. Wenk JF, Wall ST, Peterson RC, Helgerson SL, Sabbah HN, Burger M, Stander N, Ratcliffe MB, Guccione JM. A method for automatically optimizing medical devices for treating heart failure: designing polymeric injection patterns. J Biomech Eng. 2009 Dec;131(12):121011. PubMed PMID: 20524734.
3. Sack KL, Aliotta E, Choy JS, Ennis DB, Davies NH, Franz T, Kassab GS, Guccione JM. Intra-myocardial alginate hydrogel injection acts as a left ventricular mid-wall constraint in swine. Acta Biomaterialia. 2020 Jul 15; 111:170-80.
4. Sun KT, Yeatman LA, Buxton DB, Chen K, Johnson JA, Huang SC, Kofoed KF, Weismueller S, Czernin J, Phelps ME, Schelbert HR. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-carbon-11]acetate. J Nucl Med. 1998;39(2):272-80.
5. Spühler JH, Jansson J, Jansson N, Hoffman J. A finite element framework for high performance computer simulation of blood flow in the left ventricle of the human heart. KTH Royal Institute of Technology; 2015.
6. Moosavi MH, Fatouraee N, Katoozian H, Pashaei A, Camara O, Frangi AF. Numerical simulation of blood flow in the left ventricle and aortic sinus using magnetic resonance imaging and computational fluid dynamics. Computer methods in biomechanics and biomedical engineering. 2014 May 19;17(7):740-9.
7. Nguyen, C. et al. In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach. Magn Reson Med 76, 1354-1363, (2016).