4704
CMR-based Personalizing Risk Stratification for Outcomes in Dilated Cardiomyopathy: A Single-center Prospective Study.1Fuwai hospital, Beijing, China, 2Siemens Healthineers SHS AM NAM USA DI MR COLLAB, Los Angeles, CA, United States, 3Siemens Shenzhen Magnetic Resonance Ltd. SHS DI MR R&D SZN DL, Shenzhen, China
Synopsis
Keywords: Cardiomyopathy, Cardiomyopathy
In this study, we explored individual weight of CMR metrics for predicting short-term outcomes in DCM. ECV fraction performed excellent potential of clinical translation for prognosis in patients with DCM. LGE has better prognostic value than other CMR metrics for SCD and aborted SCD. LV strain may contribute to predict advanced heart failure, which not aid to the risk stratification of SCD. In this way, we developed a risk stratification model based on cardiac MR imaging including NYHA functional class, left ventricular ejection fraction, late gadolinium enhancement, and mean extracellular volume. It may improve clinical assessment and individual decision making.
Purpose
To explore individual weight of cardiac magnetic resonance (CMR) metrics to predict short-term outcomes in patients with dilated cardiomyopathy (DCM), and develop a risk algorithm for short-term outcome based on CMR biomarkers.Method
A total of 407 consecutive patients with DCM (48.1±13.8 years, 311men) who underwent cardiac magnetic resonance with a 3.0-T contrast-enhanced magnetic resonance scanner were prospectively enrolled in this study. The primary endpoint was a composite of heart failure death, sudden cardiac death (SCD), aborted SCD, and heart transplantation. Survival estimates were calculated by receiver operating characteristic analyses, competing risk regression analyses, and Cox regression analyses. Furthermore, risk factors were used to build personalizing risk stratification model.Result
During a mean follow-up of 22.0 months (range 0.5-35.5 months), 63 patients reached the primary endpoint. NYHA (hazard ratio, HR=2.327), left ventricular ejection fraction (LVEF) (HR=0.941), late gadolinium enhancement (LGE) >0.9% & ≤6.6% (HR=3.512), LGE>6.6% (HR=5.908), and mean extracellular volume (ECV) fraction≥32.8% (HR: 5.944) had a significant prognostic association with the primary end points (C statistic:0.851) (all p<0.05). Competing risk regression analyses showed that patients with mean ECV fraction ≥32.8% (SHR: 31.215, p<0.001), LGE≥5.9% (SHR: 16.421, p<0.001) or global circumferential strain ≥-5.6% (SHR: 15.218, p<0.001) had significantly shorter event-free survival due to heart failure death and heart transplantation. Patients with mean ECV fraction ≥32.8% (SHR: 8.912, p=0.003), LGE≥5.9% (SHR: 13.154, p<0.001) had significantly shorter event-free survival due to SCD or aborted SCD. The model showed great agreement in calibration plot and contributed to clinical decision making in decision curve analysis.Discussion
In this study, we comprehensively assessed the prognostic value of CMR conventional (EF, LGE) and novel parameters (native T1, ECV fraction, strain) for patients with DCM. We demonstrated that these parameters were more associated with cardiac-related death, aborted SCD and heart transplantation, comparing with the risk factors in the current guideline 6. In addition, competing risk regression analysis indicated that patients with ECVmean≥32.8% had markedly strong association with heart failure death and heart transplantation as a consequent of advanced heart failure, while LGE≥5.9% showed the highest SHR to predict SCD and aborted SCD as a consequent of malignant ventricular arrhythmia. However, patients with GCS≥-5.6%, or who accorded with the guideline criteria of ICDs implantation, had not significantly association with SCD and aborted SCD. Finally, we developed a CMR-based personalized risk stratification model to predict the 1- and 2-year probability of major cardiac adverse events. Patients who had ECVmean≥32.8% or LGE≥6.6% showed a 1.78-fold increased risk of death, aborted SCD, and heart transplantation, which showed better performance than model without novel CMR metric and current guideline.Conclusion
ECV fraction may be the best independently risk factor for the short-term outcomes in patients with DCM, surpassing LVEF and LGE. However, LGE has better prognostic value than other CMR metrics for SCD and aborted SCD. The risk stratification model we developed may be a promising non-invasive tool for decision making and prognosis.Acknowledgements
Not application.References
1. Puntmann VO, Carr-White G, Jabbour A, et al. T1-Mapping and Outcome in Nonischemic Cardiomyopathy: All-Cause Mortality and Heart Failure. JACC Cardiovasc Imaging. 2016;9(1):40-50.2. Li S, Zhou D, Sirajuddin A, et al. T1 Mapping and Extracellular Volume Fraction in Dilated Cardiomyopathy: A Prognosis Study. JACC Cardiovasc Imaging. 2021.3. Halliday BP, Cleland JGF, Goldberger JJPrasad SK Personalizing Risk Stratification for Sudden Death in Dilated Cardiomyopathy: The Past, Present, and Future. Circulation. 2017;136(2):215-231.4. Stecker EC, Vickers C, Waltz J, et al. Population-based analysis of sudden cardiac death with and without left ventricular systolic dysfunction: two-year findings from the Oregon Sudden Unexpected Death Study. J Am Coll Cardiol. 2006;47(6):1161-6.5. Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg AWellens HJ Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24(13):1204-9.6. Heidenreich PA, Bozkurt B, Aguilar D, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79(17):e263-e421.7. Køber L, Thune JJ, Nielsen JC, et al. Defibrillator Implantation in Patients with Nonischemic Systolic Heart Failure. N Engl J Med. 2016;375(13):1221-30.8. Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225-37.9. Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151-8.10. Beltrami CA, Finato N, Rocco M, et al. The cellular basis of dilated cardiomyopathy in humans. J Mol Cell Cardiol. 1995;27(1):291-305.Figures
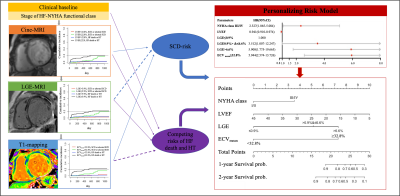
Central illustration
NYHA (hazard ratio, HR=2.327), left ventricular ejection fraction (LVEF) (HR=0.941), 0.9%<LGE≤6.6% (HR=3.512), LGE>6.6% (HR=5.908), and mean extracellular volume (ECV) fraction≥32.8% (HR: 5.944) had a significant prognostic association with the primary end points (C statistic:0.851) (all p<0.05). The risk stratification model based on CMR imaging may be potential non-invasive tool for decision making and prognosis.
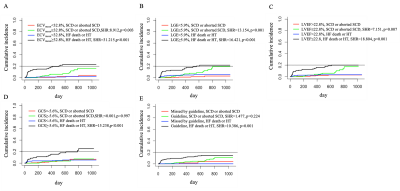
Competing risk regression analysis.
The analysis reveals increased likelihoods of both SCD, aborted SCD and HF death or heart transplantation while ECV≤32.8% (A), LGE≥5.9% (B), LVEF≤22.8% (C), respectively. GCS (D) and current guideline (E) were only associated with HF death or heart transplantation. SHR= subhazard radio, ECV= extracellular matrix volume, LGE = late gadolinium enhancement, LVEF= left ventricular ejection fraction, GCS= global circumferential strain.