4696
Probing Mitochondrial Dysfunction in Mouse Hearts with Doxorubicin-Induced Cardiotoxicity Using Ungated 4D Oxy-wavelet MRI1Department of Bioengineering, Swanson School of Engineering, University of Pittsburgh, Pittsburgh, PA, United States, 2Department of Developmental Biology, School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States, 3Rangos Research Center Small Animal Imaging Core, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 4Rangos Research Center Animal Imaging Core, Children's Hospital of Pittsburgh of UPMC, Pittsburgh, PA, United States, 5Department of Pediatrics, University of Pittsburgh, School of Medicine, Pittsburgh, PA, United States, 6Department of Chemistry, Dietrich School of Arts and Sciences, University of Pittsburgh, Pittsburgh, PA, United States, 7Department of Pediatrics, School of Medicine, University of Pittsburgh, Pittsburgh, PA, United States, 8Biomedical Imaging Research Institue, Cedars-Sinai Medical Center, Los Angeles, CA, United States
Synopsis
Keywords: Heart, Toxicity, 4D fMRI, Doxorubicin, cardiomyopathy
Cardiomyopathy caused by anti-cancer anthracyclines, such as Doxorubicin (Dox), is one major cause for long-term morbidity and mortality among cancer survivors. Patients can develop cardiomyopathy leading to heart failure decades after successful cancer treatment with Dox. There is an urgent need for early detection of sub-clinical pathogenesis of Dox-induced cardiotoxicity for early intervention before irreversible cardiac tissue damage occurs. Cellular mechanistic investigations showed that mitochondrial dysfunctions are central to the Dox-induced cardiotoxicity. We have developed a time-and-motion-resolved 4D Oxy-wavelet MRI capable of detecting early Dox-induced mitochondrial dysfunction in the heart before irreversible myocardial damage occurred.Background
Advances in cancer therapy have greatly improved patient survival. However, more than two-thirds of cancer survivors will face serious life-threatening medical conditions because of the treatments they received. Cardiomyopathy caused by anti-cancer anthracyclines, such as Doxorubicin (Dox), is one major cause for long-term morbidity and mortality among cancer survivors. Patients can develop cardiomyopathy leading to heart failure decades after successful cancer treatment with Dox. Once established, the Dox-related congestive heart failure is irreversible with a 40-50% mortality rate. There is an urgent need for early detection of sub-clinical pathogenesis of Dox-induced cardiotoxicity for early intervention before irreversible cardiac tissue damage occurs.Cellular mechanistic investigations showed that mitochondrial dysfunctions[1] are central to Dox-induced cardiotoxicity, including increased reactive oxygen species (ROS) and disturbed redox cycles primarily on the complex I of the mitochondrial respiratory chain[1-3]. We have established a motion-and-time resolved 4D functional MRI (fMRI) capable of 3D isotropic MRI time-series to simultaneously capture fast dynamic blood oxygenation level dependent (BOLD) signals in the same cardiac cine scan with both high-spatial and high-temporal resolution (voxel size: 0.00047 mm3, frame rate: 14 ms), using sub-Nyquist sparse sampling. We leverage the fact that acute [7-11] adaptation to hypoxia requires in-tact mitochondrial functions to probe mitochondrial dysfunctions, called 4D Oxy-wavelet MRI (4D-fMRI in conjunction with oscillating hypoxia challenges, analyzed by a continuous wavelet transform mimicking experimental oscillations). We tested its capability in detecting early Dox-induced mitochondrial dysfunction before myocardial damage occurred.
Methods
4D Fetal Oxy-wavelet MRI:We use a hybrid low-rank [12] and sparse [13] model to measure a dynamic BOLD image $$$\rho(\mathbf{r},t)$$$ (for spatial position $$$\mathbf{r}$$$ and time $$$t$$$) from undersampled $$$(\mathbf{k},t)$$$ -space data. The low-rank model expresses the image as the outer product of a set of $$$L$$$ basis images $$$\{\psi_\ell(\mathbf{r})\}_{\ell=1}^L$$$ and $$$L$$$ temporal functions $$$\{\varphi_\ell(t)\}_{\ell=1}^L$$$:$$\rho(\mathbf{r},t)=\sum_{\ell=1}^L\psi_\ell(\mathbf{r})\varphi_\ell(t)$$ This model exploits correlation of images over time[14] and when transform sparsity[15] of $$$\{\psi_\ell\mathbf(r)\}_{\ell=1}^L$$$ is additionally enforced during image reconstruction. This allows for fMRI with high spatiotemporal resolution; furthermore, it can assess oxygen attenuation during the same single scan. 4D-fMRI was acquired with a Bruker 7-Tesla preclinical scanner, FOV=4.5cm×3cm×2cm, isotropic voxel size 120μm×120μm×120μm, FA=10°, TR/TE=8.3ms/4.5ms, scan time=40min. During acquisition, short bursts of 3-min hypoxia (10% O2) interleaved with 3-min hyperoxia (100% O2) were supplied via a nose cone. We also developed an automated time-frequency analysis scheme for 4D oxy-wavelet MRI. Our BOLD signal is event-driven, following the oscillations of the hypoxia challenge; the event waveform resembles a square wave. Convolving the BOLD signal by template event waveforms can reveal positive or negative correlation between BOLD responses and input waveforms.
Animal Model:
C57BL6/J mice were subjected to low or high doses of Dox for 5 weeks. Low-dose (n=8) or high-dose (n=8) groups received weekly 4 mg/Kg or 8 mg/kg Dox injection for 5 weeks, with total accumulated dose of 20 mg/Kg or 40 mg/Kg respectively. Mice were subjected to 4D Oxy-wavelet MRI at baseline then weekly for 6 weeks.
Results
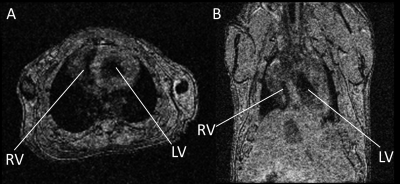
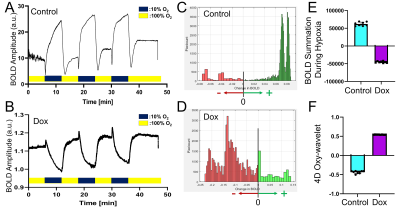
We have successfully probed in vivo acute mitochondrial dysfunctions in mouse hearts one day after Dox administration. The ungated motion-and-time-resolved 4D Oxy-wavelet MRI can acquire cine time series of 3D cardiac MRI without motion artifacts. (Fig.1). Because every time frame in the cine series is a 3D isotropic stack, it can be re-oriented to any viewing angle like the short-axis (Fig.1A) or long-axis (Fig.1B) views. The re-oriented cine series are analyzed for ejection fraction, stroke volumes, and strain.When subjected to transient short bursts of hypoxia challenges (Fig.2AB, dark blue periods), control mouse hearts (Fig.2AC & EF light blue) with intact mitochondrial functions can overcome the bursts of hypoxia to quickly adjust to a higher oxygenation state after the initial transient BOLD signal drop (Fig.2A), with most voxels showing higher than baseline BOLD levels (Fig.2C, green). The summations of overall BOLD signals during the hypoxia (Fig.2E light blue), reflecting the overall oxygenation, are positive, indicating the ability to maintain oxygen homeostasis during hypoxia. Conversely, one day after Dox treatment (Fig.2BD & EF purple), the heart showed inability to cope with hypoxia, the BOLD level stayed down during hypoxia (Fig.2B) with most voxels showing BOLD levels lower than baseline (Fig.2D, red). The summations of overall BOLD signals during the hypoxia (Fig.2E purple), reflecting the overall oxygenation, are negative, indicating the inability to maintain oxygen homeostasis during hypoxia.
Our BOLD signal is event-driven, following the oscillations of the hypoxia challenge; the event waveform resembles a square wave. The positive Oxy-wavelet indexes (Fig.2F purples) represent the positive correlation of the myocardial BOLD responses to the input even waveform (i.e. low BOLD level during hypoxia as Fig.2B), indicating passive inability to maintain oxygen homeostasis during hypoxia. On the contrary, the negative Oxy-wavelet indexes (Fig.2F blue) represent the negative correlation of the myocardial BOLD responses to the input even waveform (i.e. high BOLD level during hypoxia as Fig.2A), indicating capability to compensate for the hypoxia to maintain good oxygen homeostasis. The ability to maintain oxygenation during oscillating hypoxia challenges reflect mitochondrial functions, validated with Oroboros mitochondrial functional assays.
Conclusion
Our study showed that a single 4D Oxy-wavelet MRI scan can probe mitochondrial dysfunctions by Dox before irreversible myocardial damage occurs.Acknowledgements
No acknowledgement found.References
Wallace, K.B., V.A. Sardão, and P.J. Oliveira, Mitochondrial Determinants of Doxorubicin-Induced Cardiomyopathy. Circ Res, 2020. 126(7): p. 926-941.
2. Davies, K.J. and J.H. Doroshow, Redox cycling of anthracyclines by cardiac mitochondria. I. Anthracycline radical formation by NADH dehydrogenase. J Biol Chem, 1986. 261(7): p. 3060-7.
3. Doroshow, J.H. and K.J. Davies, Redox cycling of anthracyclines by cardiac mitochondria. II. Formation of superoxide anion, hydrogen peroxide, and hydroxyl radical. J Biol Chem, 1986. 261(7): p. 3068-74.
4. Christodoulou, A.G., et al. Fetal Brain-Heart-Placental Interactions with Acute Hypoxia Challenge in Genetic Mouse Models of Hypoplastic Left Heart Syndrome with in utero 4D Dynamic MRI. in ISMRM International Society of Magnetic Resonance in Medicine. 2019. Montreal, QC, Canada.
5. Christodoulou, A.G., et al. 4D Real-time BOLD MRI in Genetically Engineered Mouse Brains with Acute Hypoxia Challenge. in ISMRM 27th Annual Meeting. 2019. Montreal, Canada: International Soceity of Magnetic Resonance in Medicine.
6. Christodoulou, A.G., et al. Probing Mitochondrial Function in Intact Fetal Brains with Ungated 4D Oxy-wavelet MRI in an Irradiation Injury Mouse Model in International Society of Magnetic Resonance in Medicine (ISMRM). 2022. London, UK.
7. Marshall, J.M., The Joan Mott Prize Lecture. The integrated response to hypoxia: from circulation to cells. Exp Physiol, 1999. 84(3): p. 449-70.
8. Giussani, D.A., The fetal brain sparing response to hypoxia: physiological mechanisms. J Physiol, 2016. 594(5): p. 1215-30.
9. Lee, P., N.S. Chandel, and M.C. Simon, Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol, 2020. 21(5): p. 268-283.
10. Holmes, A.P., et al., Is Carotid Body Physiological O(2) Sensitivity Determined by a Unique Mitochondrial Phenotype? Front Physiol, 2018. 9: p. 562.
11. Michiels, C., Physiological and pathological responses to hypoxia. Am J Pathol, 2004. 164(6): p. 1875-82.
12. Christodoulou, A.G., et al., High-resolution cardiovascular MRI by integrating parallel imaging with low-rank and sparse modeling. IEEE Trans Biomed Eng, 2013. 60(11): p. 3083-92.
13. Zhao, B., et al., Image reconstruction from highly undersampled (k, t)-space data with joint partial separability and sparsity constraints. IEEE Trans Med Imaging, 2012. 31(9): p. 1809-20.
14. Liang, Z.-P., Spatiotemporal imaging with partially separable functions. Proc IEEE Int Symp Biomed Imaging, 2007: p. 988-991.
15. Lustig, M., D. Donoho, and J.M. Pauly, Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn Reson Med, 2007. 58(6): p. 1182-95.
Figures