4686
Impact of malignant ventricular arrhythmia on left ventricular function in dilated cardiomyopathy patients assessed by CMR feature tracking1The Second Affiliated Hospital of Nanchang University, Nanchang, China, 2GE Healthcare, Beijing, China
Synopsis
Keywords: Cardiomyopathy, Cardiomyopathy, Cardiac magnetic resonance
This study aiming at assessing the impact of malignant ventricular arrhythmia (VA+) on left ventricular (LV) function in dilated cardiomyopathy (DCM) patients using cardiovascular magnetic resonance (CMR) feature tracking (FT). 34 VA+ and 39 VA- (benign) DCM patients were measured with CMR cine imaging. Although the left ventricular ejection fraction was similar between these two groups, FT results showed the global longitudinal peak strain (GLS) was significantly lower in VA+ than in VA-. GLS had certain diagnostic value for differentiate VA+ and VA- in DCM patients. Glycosylated hemoglobin (HBA1c) was an independent determinant parameter for GLS in DCM patients.Introduction
Dilated cardiomyopathy (DCM) is characterized by the presence of left ventricular (LV) dilatation and contractile dysfunction [1]. Ventricular arrhythmias (VA) are the most feared complications in DCM[2]. Patients with DCM and malignant VA (VA+) often have a high risk of sudden cardiac death (SCD) and poor prognosis[3]. Although electrocardiogram can be used to discriminate VA+ and VA- (benign), the impact of VA on LV function in DCM patients remains unknown. The purpose of this study was to investigate if VA+ and VA- had different LV function in DCM patients using cardiac magnetic resonance (CMR) feature tracking (FT).Materials and Methods
PatientsThis retrospective study was approved by ethics review boards of our hospital and all participants agreed with the written informed consent. 73 DCM patients were involved and were divided into VA- (n=39) and VA+ (n=34) according to the electrogram examination.
MRI experiments
All CMR data were acquired on a 3T MRI system (Discovery MR750W; GE Healthcare, Milwaukee, CA, USA.) All cine images were acquired with 25 phases per cardiac cycle. Cardiac short-axis and two-, three-, and four-chamber cine images were acquired using a standard breath-held steady-state free precession cine sequence with the following parameters: slice thickness, 6mm; gap, 0mm; repetition time, 3.9msec; echo time, 1.6msec; matrix size, 256×256; field of view, 380×380mm2.
Data analysis
CMR FT analysis were performed by two experienced radiologists using CVI42 software (Circle Cardiovascular Imaging, Inc., Calgary, Canada). The optimal endocardial and epicardial contours of LV were delineated manually at the end-diastole and end-systole phases from the stack of short-axis cine images. Papillary muscles and moderator bands were diligently excluded. The global cardiac geometry and function parameters extracted including LV end-diastolic volume (LVEDV), end-systolic volume (LVESV), stroke volume (LVSV), ejection fraction (LVEF), and cardiac mass (LVM). Volume and mass measurements were indexed by body surface area (BSA). As shown in figure 1, the LV global strain parameters were quantified on short-axis cine images and long-axis cine images using the FT module of CVI42. To ensure the accuracy of the automated tracking, the observer reviewed the track and performed manual adjustments when needed. Subsequently, three-dimensional feature tracking parameters were obtained including LV global radial peak strain (GRS), global circumferential peak strain (GCS), and global longitudinal peak strain (GLS).
SPSS software was used to perform statistical analysis in this study. Two-sample t-test or the Manne Whitney U-test were used for continuous variables, and the χ2 test were used for categorical variables between two groups. Receiver operating characteristic (ROC) curve was used to evaluate the prediction performance of VA+ and VA-. The area under curve (AUC), prediction sensitivity, prediction specificity and prediction accuracy were extracted from the ROC. Multivariable linear regression analysis was used to identify the independent determinants of LV strain parameters. P < 0.05 was considered statistical significance.
Results
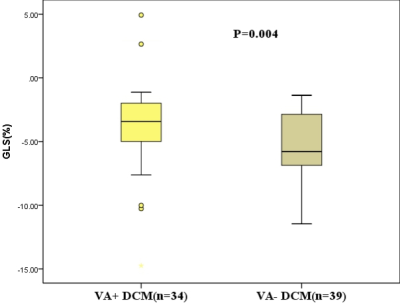
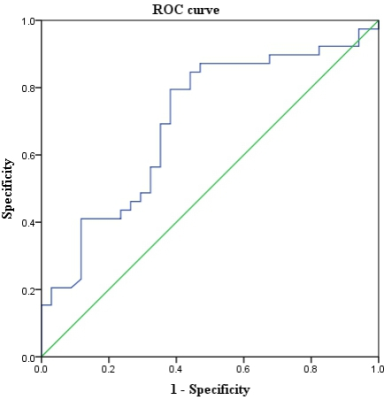
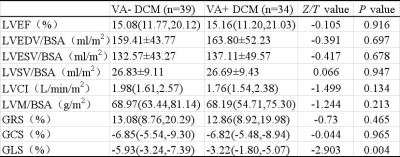
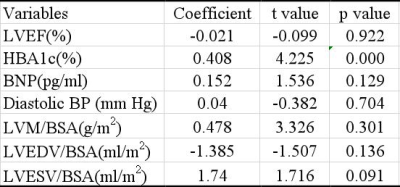
As shown in table 1 and figure 2, the GLS was significantly larger in VA+ (-3.22%) than VA- (-5.93%) DCM patients (P=0.004). The GLS presented with larger variation in VA+ DCM patients. No significant difference was observed in the other features, including LVEF, LVEDV/BSA, LVESV/BSA, LVSV/BSA, LVM/BSA, LVCI, GRS and GCS (All P>0.1). As shown in figure 3, the GLS feature presented with moderate prediction performance of VA+ and VA- with the AUC of 0.698, prediction sensitivity of 0.795, prediction specificity of 0.618 and prediction accuracy of 0.707. The results of multivariable linear regression analysis showed the glycosylated hemoglobin (HBA1c) was strong independent determinant parameter for GLS (P<0.001; Table 2).Discussion and conclusion
The main findings of this study were that 3D myocardial strain parameters (GLS) measured by CMR-FT had certain clinical value in reflecting the underlying abnormality of ventricular mechanics of DCM patients with VA+. The association between HbA1c and LV function has been reported in DCM patients[4,5]. In agreement with these studies, our results demonstrated HBA1c was an independent determinant of LV function as estimated by GLS in DCM patients. Thus, GLS and HbA1c level guided management may be able to prevent progression of HF and offer new insights into the management of DCM patients.In conclusion, GLS measured by CMR-FT had certain diagnostic value and could reflect the underlying abnormality of ventricular mechanics of DCM with MVA. HBA1c was an independent determinant of LV GLS in DCM patients.
Acknowledgements
References
[1] Weintraub G R, Semsarian C, Macdonald P. Dilated cardiomyopathy[J]. Lancet, 2017, 390(10092): 400-414.
[2] Donal E, Delgado V, Bucciarelli Ducci C, et al. Multimodality imaging in the diagnosis, risk stratification, and management of patients with dilated cardiomyopathies: an expert consensus document from the European Association of Cardiovascular Imaging[J]. Eur Heart J Cardiovasc Imaging, 2019, 20(10): 1075-1093.
[3] Halliday B P, Cleland JGF, Goldberger J J, et al. Personalizing Risk Stratification for Sudden Death in Dilated Cardiomyopathy: The Past, Present, and Future[J]. Circulation, 2017, 136(2): 215-231.
[4] Ikeda Y, Inomata T, Fujita T, et al. Higher hemoglobin A1c levels are associated with impaired left ventricular diastolic function and higher incidence of adverse cardiac events in patients with nonischemic dilated cardiomyopathy[J]. Heart Vessels, 2017, 32(4): 446-457.
[5] Shen M T, Li Y, Guo Y K, et al. Impact of type 2 diabetes mellitus on left ventricular deformation in non-ischemic dilated cardiomyopathy patients assessed by cardiac magnetic resonance imaging[J]. Cardiovasc Diabetol, 2022, 21(1): 94.
Figures