4682
Pulmonary Hypertension Detection by Cardiac Magnetic Resonance Feature Tracking in Patients with Left Heart Failure1Department of Cardiology, The Second Affiliated Hospital of Harbin Medical University, Harbin, China, HarBin, China, 2Department of Magnetic Resonance Imaging, Fuwai Hospital, State Key Laboratory of Cardiovascular Disease, National Center for Cardiovascular Diseases, Beijing, China, 3Beihang University, Beijing, China
Synopsis
Keywords: Heart, Cardiovascular, Heart failure
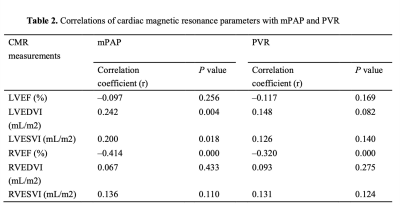
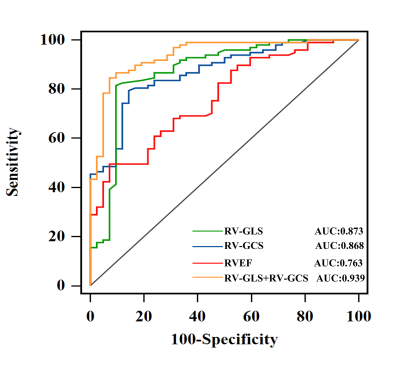
To investigate the relationship correlation of cardiac magnetic resonance (CMR) feature tracking (FT)-derived right ventricular (RV) global longitudinal strain (RV-GLS) and RV global circumferential strain (RV-GCS) with mean pulmonary artery pressure (mPAP) and pulmonary vascular resistance (PVR) to explore the diagnostic value of RV strain parameters in advanced left heart failure patients with pulmonary hypertension (PH). Study findings included as following: (a) CMR-FT RV-GLS and RV-GCS were independent risk factors for PH in patients with end-stage left heart disease, among all CMR imaging parameters; (b) Both RV-GCS (AUC = 0.868) and RV-GLS (AUC = 0.873) were valuable in predicting PH.Introduction
Pulmonary hypertension (PH) is one of the highly common diseases in advanced heart failure (HF) [1]. In the context of HF, PH is a marker of disease severity and worse prognosis [2, 3]. Right heart catheterization (RHC) is the gold standard for the diagnosis of PH by detecting mean pulmonary artery pressure (mPAP) [4, 5]. However, RHC is invasive and has the potential risk of hemorrhage, thrombosis, and other fatal complications [6]. Consequently, there is a strong need for a noninvasive alternative for hemodynamic monitoring in PH patients.Tricuspid regurgitant jet velocity detected by Doppler echocardiography is the more routinely ordinary method for noninvasive screening of PH [7]. However, inaccuracies and difficulties of such tricuspid regurgitation have been reported [8]. Cardiac magnetic resonance (CMR) is noninvasive and radiation-free and could provide an excellent examination of the right ventricle (RV) and pulmonary circulation in patients with PH [9-12]. The CMR feature tracking (CMR-FT) technique provides new insight into the assessment of PH [13-15]. However, the predictive value of CMR-FT-derived RV strain in patients with suspected PH in advanced left HF remains unknown. Therefore, this study aimed to investigate the relationship between CMR-FT-derived RV strain with RHC measured hemodynamic characteristics, and evaluate the potential of RV strain as a noninvasive alternative in identifying PH in patients with advanced left HF.Methods:
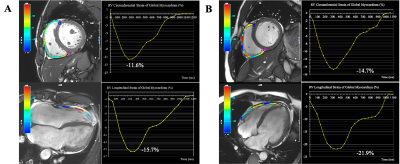
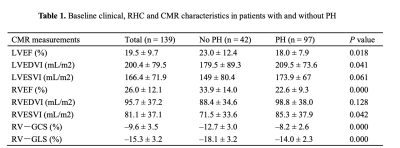
Between February 2017 and November 2019, we retrospectively enrolled 139 adult patients with advanced HF consecutively admitted to our Institute for RHC and heart transplantation evaluation due to either ischemic heart disease, non-ischemic valvular disease, or non-ischemic cardiomyopathy. All enrolled patients underwent CMR and RHC simultaneously (the interval between CMR and RHC did not exceed 48 hours). CMR images were acquired using a phased-array cardiovascular coil with electrocardiographic and respiratory gating during breath-holds on 3.0 Tesla scanners (Discovery MR750, GE Healthcare, Chicago, IL, USA; Ingenia CX, Philips Healthcare, the Netherlands). The RV myocardial strain parameters were acquired on short-axis and 4-chamber view cine images, including RV global longitudinal strain (RV-GLS) and RV global circumferential strain (RV-GCS). The endocardial and epicardial borders of the RV wall were delineated semi-automatically on end-diastole images. Based on the delineations, the strain parameters were calculated via the software package, and representative cases are shown in Figure 1. Continuous variables were compared using an independent-samples t-test or Mann-Whitney U test. Categorical data were compared using the χ2 or Fisher’s exact test. The relationships between CMR parameters and mPAP and PVR were tested using linear regression analysis with Spearman correlation coefficient. Logistic regression analysis was used to identify independent predictors of PH. Receiver operating characteristic (ROC) curve analysis was used to evaluate the ability of different RV strain parameters to diagnose PH; the areas under the ROC curve (AUC) were compared with DeLong test. All statistical analyses were conducted using IBM SPSS Statistics 26.0 software (IBM Corp., Armonk, NY, USA) and MedCalc 15.0 (MedCalc Software). A two-tailed P-value <0.05 was considered statistically significant.Results: The RV-GLS and RV-GCS were significantly impaired in the PH group when compared with the no PH group (−14.0 ± 2.3% vs. −18.1 ± 3.2%, p = 0.000 and −8.2 ± 2.6% vs. −12.7 ± 3.0%, p = 0.000, respectively) (Table 1). Invasive mPAP was significantly correlated with RV-GCS (r = 0.547, P = 0.000) and RV-GLS (r = 0.559, P = 0.000). RHC-calculated PVR was also correlated with RV-GCS (r = 0.460, P = 0.000) and RV-GLS (r = 0.682, P = 0.000) (Table 2). RV-GLS and RV-GCS were independent predictors of PH in advanced heart failure patients (AUC= 0.873 and 0.868, respectively). Their combined AUC achieved 0.939, which was significantly higher than respective values (both P < 0.05) (Figure 2).Discussion
In this study, we showed that in advanced HF patients, RV global strain significantly reduced in the PH group compared with the no PH group. Furthermore, RV-GLS and RV-GCS were significantly correlated with mPAP (r = 0.559 and r = 0.547, respectively) and PVR (r = 0.682 and r = 0.460, respectively); the RV global strain was significantly correlated with the right ventricular afterload (mPAP and PVR). Since the wall of the RV is much thinner than the LV’s, the RV does not tolerate systemic arterial pressures [16]. Therefore, RV has been more sensitive to stress caused by elevating pulmonary artery pressure and PVR. A study conducted that speckle tracking echocardiography demonstrated that persistent RV pressure overload directly affects RV longitudinal strain [17]. In a small study of 21 chronic thromboembolic pulmonary hypertension, the CMR-FT RV-GLS and RV-GCS could improve patients with mPAP < 30 mmHg [18]. These indicated that an elevation in the afterload could be one of the important mechanisms of an impaired RV strain in left heart disease. Also, some studies have found that after the RV afterload reduced, the RV myocardial strain could be improved within a certain range [19, 20].Therefore, CMR-FT-derived RV strain provide a noninvasive alternative in identifying PH in patients with advanced left HF. Thus, ultrasonography may alternative method for investigating pulmonary function using a safe, noninvasive technique that is easy to access.Conclusions
RV-GLS and RV-GCS could identify PH in patients with advanced heart failure.Acknowledgements
NoneReferences
1. Guazzi M, Naeije R. Pulmonary Hypertension in Heart Failure: Pathophysiology, Pathobiology, and Emerging Clinical Perspectives. J Am Coll Cardiol. 2017;69(13):1718-1734.
2. Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. J Am Coll Cardiol. 1999;34(6):1802-1806.
3. Shah A, Claggett B, Sweitzer N, et al. Cardiac structure and function and prognosis in heart failure with preserved ejection fraction: findings from the echocardiographic study of the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) Trial. Circulation Heart failure. 2014;7(5):740-751.
4. Armstrong GT, Joshi VM, Zhu L, et al. Increased tricuspid regurgitant jet velocity by Doppler echocardiography in adult survivors of childhood cancer: a report from the St Jude Lifetime Cohort Study. J Clin Oncol. 2013;31(6):774-781.
5. Chan IP, Weng MC, Hsueh T, et al. Prognostic value of right pulmonary artery distensibility in dogs with pulmonary hypertension. J Vet Sci. 2019;20(4):e34.
6. Shah S, Boyd G, Pyne CT, et al. Right heart catheterization using antecubital venous access: feasibility, safety and adoption rate in a tertiary center. Catheter Cardiovasc Interv. 2014;84(1):70-74.
7. Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37(1):67-119.
8. Janda S, Shahidi N, Gin K, et al. Diagnostic accuracy of echocardiography for pulmonary hypertension: a systematic review and meta-analysis. Heart. 2011;97(8):612-622.
9. Saba TS, Foster J, Cockburn M, et al. Ventricular mass index using magnetic resonance imaging accurately estimates pulmonary artery pressure. Eur Respir J. 2002;20(6):1519-1524.
10. Lungu A, Swift AJ, Capener D, et al. Diagnosis of pulmonary hypertension from magnetic resonance imaging-based computational models and decision tree analysis. Pulm Circ. 2016;6(2):181-190.
11. Swift A, Lungu A, Walker H, et al. Improved diagnostic accuracy of MRI in patients with suspected pulmonary hypertension with combined right ventricle and pulmonary artery metrics. European Respiratory Journal. 2015;46(suppl 59):PA2457.
12. Kjellström B, Lindholm A, Ostenfeld E. Cardiac Magnetic Resonance Imaging in Pulmonary Arterial Hypertension: Ready for Clinical Practice and Guidelines? Curr Heart Fail Rep. 2020;17(5):181-191.
13. Padervinskienė L, Krivickienė A, Hoppenot D, et al. Prognostic Value of Left Ventricular Function and Mechanics in Pulmonary Hypertension: A Pilot Cardiovascular Magnetic Resonance Feature Tracking Study. Medicina (Kaunas, Lithuania). 2019;55(3).
14. de Siqueira M, Pozo E, Fernandes V, et al. Characterization and clinical significance of right ventricular mechanics in pulmonary hypertension evaluated with cardiovascular magnetic resonance feature tracking. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2016;18(1):39.
15. Leong K, Howard L, Lo Giudice F, et al. MRI Feature Tracking Strain in Pulmonary Hypertension: Utility of Combined Left Atrial Volumetric and Deformation Assessment in Distinguishing Post- From Pre-capillary Physiology. Front Cardiovasc Med. 2022;9:787656.
16. Kosiborod M, Wackers FJ. Assessment of right ventricular morphology and function. Semin Respir Crit Care Med. 2003;24(3):245-262.
17. Puwanant S, Park M, Popović ZB, et al. Ventricular geometry, strain, and rotational mechanics in pulmonary hypertension. Circulation. 2010;121(2):259-266.
18. Kawakubo M, Yamasaki Y, Kamitani T, et al. Clinical usefulness of right ventricular 3D area strain in the assessment of treatment effects of balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension: comparison with 2D feature-tracking MRI. Eur Radiol. 2019;29(9):4583-4592.
19. Waziri F, Ringgaard S, Mellemkjær S, et al. Long-term changes of right ventricular myocardial deformation and remodeling studied by cardiac magnetic resonance imaging in patients with chronic thromboembolic pulmonary hypertension following pulmonary thromboendarterectomy. Int J Cardiol. 2020;300:282-288.
20. Wright L, Negishi K, Dwyer N, et al. Afterload Dependence of Right Ventricular Myocardial Strain. J Am Soc Echocardiogr. 2017;30(7):676-684.e671.
Figures