4680
Myocardial strain and strain rate reveal changes on regional cardiac function shortly post chemotherapy
El-Sayed H. Ibrahim1 and John Charlson1
1Medical College of Wisconsin, Milwaukee, WI, United States
1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Keywords: Heart, Cancer
Anthracycline chemotherapy is the mainstay of cancer treatment. While the most important late, potentially permanent, side effect of chemotherapy is cardiotoxicity, the short-term acute effects of chemotherapy on cardiac function are not well elucidated. In this study, we used advanced cardiac MRI to assess subclinical changes in cardiac function immediately post chemotherapy treatment. The results demonstrated that strain and strain rate are sensitive imaging parameters that allow for early detection of changes in systolic and diastolic myocardial contractility immediately post chemotherapy despite slight changes in global cardiac function and tissue characterization.Introduction
Anthracycline chemotherapy is the mainstay of cancer treatment in both common, e.g., breast cancer, and rare, e.g., sarcoma, cancers. While the most important late, potentially permanent, side effect of chemotherapy is cardiotoxicity, which if left untreated may progress to heart failure (HF), the short-term acute effects of chemotherapy on cardiac function are not well elucidated. The current paradigm for cardiotoxicity detection and management relies primarily upon assessment of global heart function, e.g., ejection fraction (EF). However, cardiac injury can occur without a clear change in EF due to the heart’s compensatory mechanism to maintain cardiac output in the face of regional function abnormalities. Therefore, there is a need for identifying new methods capable of early detection of subclinical cardiac changes to allow for selecting individuals who could benefit from therapeutic interventions to prevent HF development. In this study, we used advanced cardiac MRI to assess subclinical changes in cardiac function immediately post chemotherapy treatment.Methods
In this IRB-approved study, five cancer patients (3 females and 2 males; 3 sarcoma and 2 breast cancer) scheduled for chemotherapy were included in the study. The patients underwent pre-treatment cardiac MRI exams, and three of them underwent repeated post-treatment exams (within 2-weeks) to evaluate acute changes in global and regional cardiac function post treatment. The MRI exams were conducted on a GE 3T MRI scanner to acquire long-axis and short-axis cine images covering the whole heart. Optimized imaging parameters for the cine sequence were: FIESTA acquisition, repetition time (TR) = 3.6 ms, echo time (TE) = 1.3 ms, flip angle = 55°, views per segment = 14, # averages = 1, matrix = 256×256, slice thickness = 8 mm, and readout bandwidth = 488 Hz/pixel. T1 and T2 mapping sequences were also included in the exam. Optimized imaging parameters for T1 mapping were: 5(3)3 MOLLI sequence, FIESTA acquisition, TR = 2.9 ms, TE = 1.3 ms, flip angle = 35°, slice thickness = 8 mm, matrix = 160×148, FOV = 360×360 mm2, # averages = 1, and readout bandwidth = 977 Hz/pixel. Optimized imaging parameters for T2 mapping were: multi-echo spin-echo sequence, TR = 895 ms, TE = 11 − 77 ms (4 echoes with 22 ms increments), echo train length (ETL) = 16, flip angle = 90°, slice thickness = 8 mm, matrix = 180×180, FOV = 360×360 mm2, # averages = 1, readout bandwidth = 651 Hz/pixel. The cine images were analyzed using the Circle cvi42 software to generate measures of global cardiac function (ventricular ejection fraction (EF), indexed end-diastolic (EDV), end-systolic (ESV), and mass). The tissue tracking technique was used to generate regional cardiac function parameters as follows: global longitudinal, circumferential, and radial peak systolic strains (GLS, GCS, GRS); longitudinal, circumferential, and radial peak diastolic strain rates (LSR, CSR, RSR); longitudinal, circumferential, and radial time-to-peak systolic strain (normalized to the cardiac cycle length (RR); LTTPS, CTTPS, RTTPS); and basal to apical LV torsion. Finally, global T1 and T2 measurements were calculated via exponential curve fittings of the signal intensity from the acquired images. Statistical analysis was conducted to compare measurements pre- and post-treatment.Results
Global cardiac function measurements are summarized in Table 1. The patients had slight decrease in LV EF (60±4% vs 65±3%) and increases in EDV, ESV and mass post-chemotherapy (91±9 ml/m2, 36±4 ml/m2, 57±8 g/m2 vs 80±19 ml/m2, 28±9 ml/m2, 55±8 g/m2, respectively). There were minimal changes in global cardiac function parameters in the RV. Regional cardiac function measurements are summarized in Table 2. All systolic strain (Figure 1) and diastolic strain rate (Figure 2) measurements slightly increased (in absolute value) post-treatment. GLS, GCS, GRS = -17.3±1.5%, -19.3±2.1%, 34.7±5.9 vs -15.2±2.3%, -18.2±4%, 31.8±9.3%, respectively. LSR, CSR, RSR = 0.9±0.3s-1, 1±0.3s-1, -1.9±0.7s-1 vs 0.7±0.3 s-1, 0.9±0.3 s-1, -1.8±0.9 s-1, respectively. LV torsion showed slight decrease post-treatment (0.7±0.2°/cm vs 0.8±0.5°/cm). Time-to-peak-systolic strains were maintained post-treatment. Global T1 and T2 measurements were maintained post-treatment: 1259±24 ms and 49±2.1 ms post-treatment vs 1254±57 ms and 49±1.7 pre-treatment, respectively.Discussion and Conclusions
Cardiac MRI is a valuable imaging modality for detailed evaluation of transient changes in cardiac function immediately post chemotherapy treatment in cancer patients. Despite minimal changes in issue characterization (global T1 and T2 measurements) during the short duration between the pre-treatment and post-treatment exams, cardiac functional parameters revealed changes in heart contractility. Although global cardiac function parameters showed slight deterioration (decreased EF and increased volumes and mass), regional cardiac function parameters, represented by strain and strain rate, showed increased contractility post treatment, which may be attributed to ventricular remodeling as an acute response to injury from chemotherapy. In conclusion, cardiac MRI strain and strain rate are sensitive imaging parameters that allow for early detection of subclinical changes in systolic and diastolic myocardial contractility immediately post chemotherapy, which would allow for identifying patients at-risk with opportunity for prompt intervention to avoid cardiac complications and development of heart failure.Acknowledgements
Study supported by MCW Cancer Center and GE Healthcare.References
1. KW Zhang et al. JACC Cardiovasc Imaging 2018;11:1059-68
2. BC Drafts et al. JACC Cardiovasc Imaging 2013;6:877-85
3. O Toro-Salazar et al. Circ Cardiovasc Imaging 2013;6:873-80
4. EH Ibrahim. Heart Mechanics. MRI. CRC Press, 2017
Figures
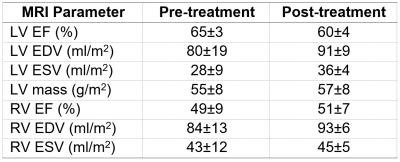
Table 1.
Global cardiac function measurements (mean±SD). Abbreviations: EF, ejection fraction; EDV, end-diastolic
volume index; ESV, end-systolic volume index.
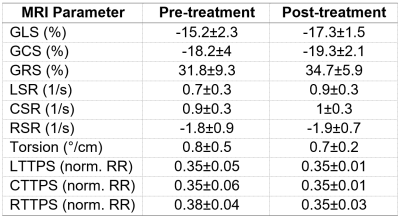
Table 2.
Regional cardiac function measurements (mean±SD). Abbreviations: GLS, GCS, GRS: global longitudinal,
circumferential, and radial peak systolic strains; LSR, CSR, RSR: longitudinal,
circumferential, and radial peak diastolic strain rates; LTTPS, CTTPS, RTTPS:
longitudinal, circumferential, and radial time-to-peak strain (normalized to
the cardiac cycle length (RR)).
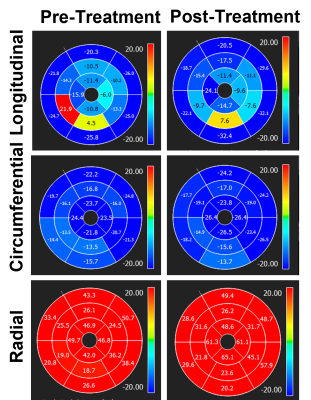
Figure 1. Peak-systolic
strain measurements (unit =%) pre- and post-treatment in a cancer patient
treated with chemotherapy. Note increases in (absolute) strain measurements
immediately post chemotherapy treatment, which reflect undergoing ventricular remodeling.
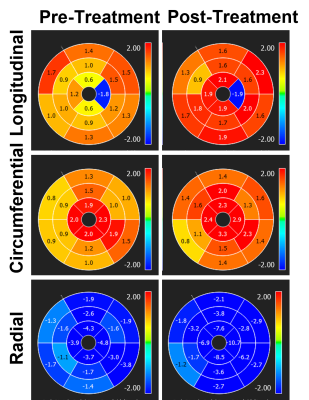
Figure 2. Peak-diastolic
strain rate measurements (unit = 1/s) pre- and post-treatment in a cancer
patient treated with chemotherapy. Note increases in (absolute) strain rate
measurements immediately post chemotherapy treatment, which reflect undergoing
ventricular remodeling.
DOI: https://doi.org/10.58530/2023/4680