4670
Assessment of pulmonary function in pulmonary embolism using phase-resolved functional pulmonary MRI (PREFUL-MRI) under free breathing1Department of Radiology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China, 2MR Scientific Marketing, Siemens Healthcare, Beijing, China, 3Institute for Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany
Synopsis
Keywords: Lung, Perfusion
This study investigated the difference in pulmonary function (including perfusion and ventilation defects) using PREFUL-MRI between pulmonary embolism patients and healthy volunteers. Three coronal slices were acquisitor with MAGNETOM Prisma 3T MR scanner per subject. The results showed that perfusion and ventilation defect percentages derived from PREFUL-MRI in PE patients were higher than in healthy subjects. And the PREFUL-MRI parameters showed a strong correlation with spirometry parameters in PE patients. Our study suggests that PREFUL-MRI allows for the quantification of lung function under free breathing and non-contrast in PE patients, making this a promising tool for future monitoring of patientsIntroduction
Pulmonary embolism (PE) is a common cardiovascular emergency that lacks clinical features and may endanger life. Early diagnosis and treatment of PE potentially can reverse right ventricular failure 1,2. Single photon emission computed tomography (SPECT) remains the most preferred and effective method to diagnose pulmonary embolism (PE). However, SPECT requires the injection of a radiopharmaceutical, resulting in exposure to gamma radiation, and dynamic contrast-enhanced MRI (DCE-MRI) requires the administration of gadolinium-based intravenous contrast agents. Postprocessing of dynamic free-breathing non-contrast enhanced 1H lung MRI allows assessment of lung perfusion and ventilation. Phase-resolved functional lung MRI (PREFUL-MRI) using a 1H lung MRI Fourier decomposition technique can be used to quantify the lung ventilation and perfusion parameters 3,4. Recent studies have demonstrated that the assessment of pulmonary perfusion derived from PREFUL-MRI is comparable to SPECT and DCE-MRI in patients with chronic obstructive pulmonary disease (COPD) 5,6. However, so far its potential effect to quantify lung perfusion and ventilation in PE patients has not been validated. Therefore, our study aims to compare pulmonary perfusion and ventilation using PREFUL-MRI in PE patients and healthy volunteers and to evaluate the correlation of perfusion and ventilation defect with spirometry parameters in PE patients.Method
9 patients with PE and 11 healthy controls were recruited. And all subjects gave written informed consent. Three coronal slices for each patient were acquired with MAGNETOM Prisma 3T MR scanner (Siemens Healthcare, Erlangen, Germany) using a spoiled gradient echo sequence. The parameters of 2D FLASH are as follows: FOV =500 × 500 mm2, matrix = 128 × 128, slice thickness =15 mm, TR/TE=2.5ms /0.9 ms, flip angle = 5°, bandwidth =1500 Hz/pixel, temporal resolution = 256 ms, total acquisition duration 36 s. For each patient, 280 measurements per slice were obtained. The postprocessing of PREFUL-MRI was calculated with an MR lung prototype (Siemens Healthcare, Erlangen, Germany). Spirometry parameters include forced expiratory volume in 1 second (FEV 1), forced vital capacity (FVC), reserve volume (RV), total lung capacity (TLC), and inspiratory capacity (IC). The continuous variables between the two groups were compared using Mann–Whitney U-test. The obtained parameters were correlated using Spearman’s correlation coefficient (r).Results
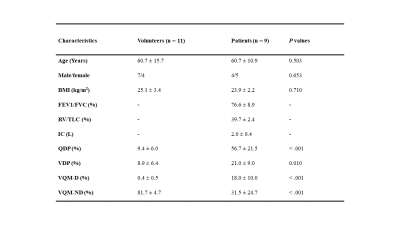
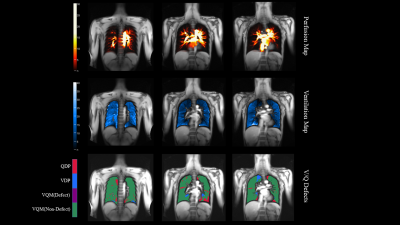
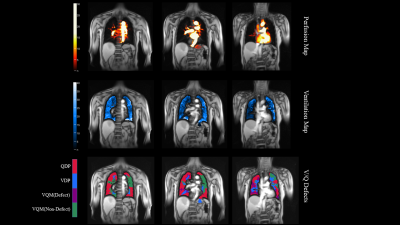
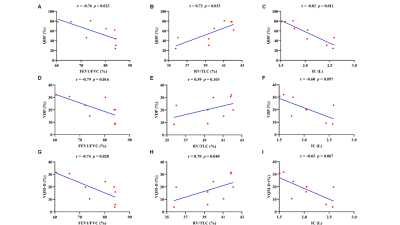
The detailed demographics of 20 subjects were presented in Table 1. Additionally, the representative PREFUL-MRI imaging of healthy subjects and PE patient were shown in Fig 1 and Fig 2 respectively. The perfusion defect percentage (QDP), ventilation defect percentage (VDP), and ventilation and perfusion match defect percentage (VQM-D) in the PE patients were significantly higher than in healthy volunteers (P < 0.001, P = 0.010, P < 0.001). PREFUL-QDP showed a negative correlation with FEV1/FVC and IC (r = -0.76, p = 0.023; r = -0.81, p = 0.011). On the contrary, PREFUL-QDP was positively correlated with RV/TLC (r = 0.72, p = 0.033). Meanwhile, the similar results were acquired in PREFUL-VDP and PREFUL-VQM-D (PREFUL-VDP vs. FEV1/FVC, r = -0.79, p = 0.016; PREFUL-VDP vs. RV/TLC, r = 0.59, p = 0.103; PREFUL-VDP vs. IC, r = -0.60, p = 0.097; PREFUL-VQM-D vs. FEV1/FVC, r = -0.74, p = 0.028; PREFUL-VQM-D vs. RV/TLC, r = 0.70, p = 0.040; PREFUL-VQM-D vs. IC, r = -0.65, p = 0.067) as shown in Fig 3.Discussion
This study demonstrated that the assessment of lung perfusion and ventilation defects was significantly increased in PE patients. And the PREFUL-QDP, PREFUL-VDP, and PREFUL-VQM-D showed a strong correlation with spirometry parameters. For the diagnosis and evaluation of PE disease, the SPECT and DCE-MRI required the patients to be exposed to radiation for a long time or administration of contrast agents. PREFUL-MRI allows patients to assess pulmonary perfusion and ventilation under free breathing without contrast agents. Furthermore, PREFUL-MRI correlated with spirometry parameters, making it a potentially useful tool for further investigation of the early diagnosis and treatment evaluation of PE in future studies.Conclusion
The PREFUL-MRI is a free-breathing, radiation-free and effective diagnostic tool for quantifying lung perfusion and ventilation defects in PE disease.Acknowledgements
No acknowledgement found.References
1. Roy PM, Meyer G, Vielle B, et al. Appropriateness of diagnostic management and outcomes of suspected pulmonary embolism. Annals of internal medicine 2006; 144(3): 157-64.
2. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). European heart journal 2008; 29(18): 2276-315.
3. Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of fourier decomposition in proton MRI. Magnetic resonance in medicine 2009; 62(3): 656-64.
4. Voskrebenzev A, Gutberlet M, Klimeš F, et al. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magnetic resonance in medicine 2018; 79(4): 2306-14.
5. Behrendt L, Voskrebenzev A, Klimeš F, et al. Validation of Automated Perfusion-Weighted Phase-Resolved Functional Lung (PREFUL)-MRI in Patients With Pulmonary Diseases. Journal of magnetic resonance imaging : JMRI 2020; 52(1): 103-14.
6. Kaireit TF, Voskrebenzev A, Gutberlet M, et al. Comparison of quantitative regional perfusion-weighted phase resolved functional lung (PREFUL) MRI with dynamic gadolinium-enhanced regional pulmonary perfusion MRI in COPD patients. Journal of magnetic resonance imaging : JMRI 2019; 49(4): 1122-32.
Figures