4667
Metronome-paced Tachypnea-Induced Dynamic Hyperinflation on Pulmonary cine MRI in Chronic Obstructive Pulmonary Disease: SPIROMICS HF Study1Columbia University, New York, NY, United States, 2University of California, Los Angeles, Los Angeles, CA, United States, 3University of Iowa, Iowa City, IA, United States, 4Johns Hopkins University, Baltimore, MD, United States, 5University of Michigan, Ann Arbor, MI, United States, 6University of Utah, Salt Lake City, UT, United States, 7Hannover Medical School, Hanover, Germany, 8University of Wisconsin, Madison, WI, United States
Synopsis
Keywords: Lung, Lung, chronic obstructive pulmonary disease, pulmonary cine MRI, metronome-paced tachypnea, dynamic hyperinflation, transfer learning, unsupervised domain adaptation
Metronome-paced tachypnea-induced dynamic hyperinflation of the lungs is typically assessed with spirometry; this method does not measure the dynamic change in lung volume over time. We implemented metronome-paced tachypnea with cardiopulmonary cine MRI. We developed an automated lung area segmentation algorithm using unsupervised domain adaptation (UDA) to measure dynamic hyperinflation in a SubPopulations and Intermediate Outcomes In COPD and Heart Failure Study (SPIROMICS HF) sample (n=65). Preliminary results showed that the integration of metronome-paced tachypnea and pulmonary cine MRI can quantify dynamic hyperinflation; dynamic hyperinflation identified on MRI appears to be associated with COPD severity.Purpose
Dynamic hyperinflation is a major cause of dyspnea and impaired exercise tolerance in chronic obstructive pulmonary disease (COPD)1. Metronome-paced tachypnea is a validated technique to induce dynamic hyperinflation in COPD, which has previously only been assessed with spirometry1. But spirometry does not provide dynamic assessment of cardiopulmonary function. We therefore implemented metronome-paced tachypnea with continuous, 3 frames per second cardiopulmonary cine MRI to measure lung volumes during respiration and blood flow in a multicenter study. Using unsupervised domain adaptation (UDA)2,3, we developed an automated lung area segmentation algorithm for lung volumes on cardiopulmonary cine MRI to measure dynamic hyperinflation and hypothesized that the method yields accurate and reproducible measure of dynamic hyperinflation that vary by COPD severity.Methods
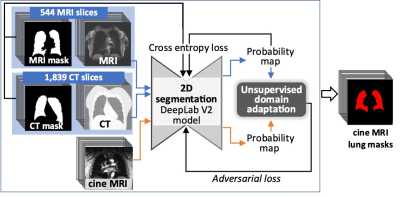
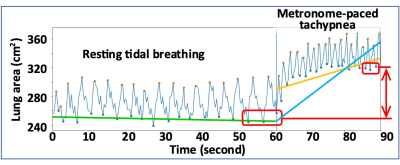
The SubPopulations and InteRmediate Outcome Measures In COPD (SPIROMICS) is a prospective cohort study that enrolled 2,981 participants with 20+ packyears of smoking with the goals of identifying new COPD subgroups and intermediate markers of disease progression4. The SPIROMICS Heart Failure (HF) substudy is evaluating cardiopulmonary interactions with MRI among 600 SPIROMICS participants. COPD status was defined by Global Initiative for Obstructive Lung Disease (GOLD) severity with GOLD 0 (individuals ‘at risk’ for COPD) stratified by symptoms based upon a COPD Assessment Test score >=10. The current report is based upon preliminary results from one site. During MRI acquisition with a cine ultra-fast coronal gradient-echo sequence centered at the trachea, participants performed resting tidal breathing for 60 seconds followed by 30 seconds of metronome-paced tachypnea at 40 breaths/min. To automatically segment the full lung mask from 2D pulmonary cine MRI, we developed a transfer learning framework using UDA. Previously, we had semi-automatically segmented pulmonary MRI and CT scans from an independent COPD study and SPIROMICS using sliceOmatic (Figure 1)5. An encoder-decoder-based deep learning model (i.e., DeepLab V2)6 was trained on 544 MRI slices and 1,839 CT slices along with lung masks and was further fine-tuned on pulmonary cine MRIs via UDA (Figure 1)2,3. Incorporated with adversarial training, the model was trained to generate similar entropy maps from different domains, which indicates a similar confidence level on segmentation. The final model was selected based on validation results on labeled CT and MRI as well as the prediction confidence on unlabeled cine MRI. Dynamic hyperinflation was calculated as the increase in end-expiratory lung area from resting tidal breathing to the end of metronome-paced tachypnea1. An automatic spike detection algorithm was utilized to automatically find the spikes (end of inspiration lung area and end of expiration lung area) of the lung area-time plots (Figure 2). Additionally, to explore potential new parameters to characterize dynamic hyperinflation, linear regression lines were fitted on the end of expiration lung areas for tidal breathing (Figure 2, green line) and metronome-paced tachypnea (Figure 2: blue and yellow lines with and without a knot tying to tidal breathing volume, respectively).Results
The 65 participants were (mean±SD) 65±10 years old, 37% were male, and all had smoked >20 packyears; 10 (15%) were asymptomatic and 9 (14%) were symptomatic without COPD, 25 (38%) had mild-moderate COPD and 6 (9%) had severe COPD. Compared to manual segmentation of lung masks on pulmonary cine MRI, the UDA model had high accuracy (segmented lung area r=0.997, P<0.001). There were large differences in end-expiratory lung area during tidal breathing (green line in Figure 2) by COPD status (P<0.001). Metronome-paced tachypnea resulted in an average absolute increase in end-expiratory lung area (red line in Figure 2) of 34 cm2, which was higher with greater COPD severity (P=0.0495). Metronome-paced tachypnea also resulted in an average rate of rise in end-expiratory lung area (blue line in Figure 2) from tidal volume of 1.7 cm2/sec, which was also higher with greater COPD severity (P=0.046). Metronome-paced tachypnea resulted in an average increase in end-expiratory lung area (yellow line in Figure 2) of 0.27 cm2/sec, but this did not differ by COPD status (P=0.21).Conclusion
These preliminary results showed that the integration of metronome-paced tachypnea and pulmonary cine MRI is a novel technique that can quantify dynamic hyperinflation and that dynamic hyperinflation measured on cine pulmonary MRI worsens with COPD severity. Future evaluation is needed to compare the value of determining the absolute increase in volume versus the rate of increase of volume during metronome breathing. Comparing to spirometry, dynamic imaging methods provide opportunities to explore using new parameters to quantify dynamic hyperinflation.Acknowledgements
Funding NIH/NHLBI R01-HL093081, R01-HL121270, "The Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) was supported by contracts from the NIH/NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca; Bellerophon Therapeutics; Boehringer-Ingelheim Pharmaceuticals, Inc; Chiesi Farmaceutici SpA; Forest Research Institute, Inc; GSK; Grifols Therapeutics, Inc; Ikaria Inc; Nycomed GmbH; Takeda Pharmaceutical Company; Novartis Pharmaceuticals Corporation; Regeneron Pharmaceuticals, Inc; and Sanofi."References
1. Calligaro GL, Raine RI, Bateman ME, Bateman ED and Cooper CB. Comparing dynamic hyperinflation and associated dyspnea induced by metronome-paced tachypnea versus incremental exercise. COPD. 2014;11:105-12.
2. Vu T, Jain H, Bucher M, Cord M and Perez P. Advent: Adversarial entropy minimization for domain adaptation in semantic segmentation. Proceedings of the IEEE/CVF Conference on Computer Vision and Pattern Recognition 2019:2517-2526.
3. Zeng, G., Schmaranzer, F., Lerch, T.D., Boschung, A., Zheng, G., Burger, J., Gerber, K., Tannast, M., Siebenrock, K., Kim, Y.J. and Novais, E.N., Entropy guided unsupervised domain adaptation for cross-center hip cartilage segmentation from MRI. In International Conference on Medical Image Computing and Computer-Assisted Intervention. 2020: 447-456.
4. Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, Kanner R, Kleerup E, Martinez FJ and Woodruff PG. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS). Thorax. 2014;69:492-495.
5. Zhang X, Angelini ED, Haghpanah FS, Laine AF, Sun Y, Hiura GT, Dashnaw SM, Prince MR, Hoffman EA, Ambale-Venkatesh B, Lima JA, Wild JM, Hughes EW, Barr RG and Shen W. Quantification of lung ventilation defects on hyperpolarized MRI: The Multi-Ethnic Study of Atherosclerosis (MESA) COPD study. Magn Reson Imaging. 2022;92:140-149.
6. Chen L-C, Papandreou G, Kokkinos I, Murphy K and Yuille AL. Deeplab: Semantic image segmentation with deep convolutional nets, atrous convolution, and fully connected CRFs. IEEE transactions on pattern analysis and machine intelligence. 2017;40:834-848.
Figures