4666
Dynamic single slice 2D coronal lung MRI detects metronome-paced tachypnea (MPT) - induced hyperinflation in COPD patients1Institute of Diagnostic and Interventional Radiology, Hannover Medical School, Hannover, Germany, 2Biomedical Research in Endstage and Obstructive Lung Disease Hannover (BREATH), German Center for Lung Research (DZL), Hannover, Germany, 3Department of Radiology, Weill Cornell Medical College, New York, NY, United States, 4Department of Radiology, Columbia College of Physicians and Surgeons, New York, NY, United States, 5Departments of Medicine and Physiology, David Geffen School of Medicine, Los Angeles, CA, United States, 6Department of Medicine, New York-Presbyterian/Columbia University Irving Medical Center, New York, NY, United States, 7Department of Respiratory Medicine, Hannover Medical School, Hannover, Germany, 8Fraunhofer Institute of Toxicology and Experimental Medicine, Hannover, Germany
Synopsis
Keywords: Lung, Lung, COPD
Metronome-paced tachypnea (MPT) is a technique where a patient breathes rapidly with a fixed frequency (40 breaths / minute). MPT induces dynamic hyperinflation and allows investigation of potential stress-driven effects on ventilation parameters. Fifteen patients with chronic obstructive pulmonary disease (COPD) and thirty healthy subjects underwent MRI with a 2D coronal lung MR image acquired repeatedly with a temporal resolution of 200 ms during resting tidal breathing (RTB) and MPT. The decrease of fractional ventilation (FV) and the increase of end-expiratory lung area (EELA) during MPT was significantly higher in COPD patients compared to respective healthy subjects.Introduction
Hyperinflation in chronic obstructive pulmonary disease (COPD) patients worsens on exertion / exercise when breathing frequency increases.1,2 Fast breathing, paced at 40 breaths per minute using a metronome, referred to as metronome-paced tachypnea3 (MPT), can be performed during MRI. MPT has been shown to induce dynamic hyperinflation in COPD patients and can be used to simulate exercise during MRI.3 Until now, the potential effects of MPT on pulmonary ventilation function derived by MRI in COPD patients compared to healthy subjects have not been assessed. Therefore, the purpose of this study was to assess repeatability and to compare global lung ventilation during resting tidal breathing (RTB) and MPT using a 2D single-slice time series of coronal spoiled gradient-echo (SPGR) images in COPD patients and healthy subjects.Methods
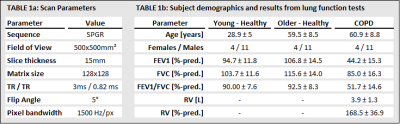
Fifteen patients (11 males, 4 females; age range 40-77 years) with stable, moderate to severe COPD (post-bronchodilator forced expiratory volume in 1 s (FEV1) < 80 %, 400 μg salbutamol) and hyperinflation (residual volume (RV) > 135 % of predicted normal) and thirty healthy subjects (22 males, 8 females; age range 21-78 years) have been examined in an ongoing single center study. The healthy subjects were divided by age into two groups: below and above 40 years. The 15 older-healthy subjects were age and sex matched to the COPD patients. All COPD patients underwent spirometry and body plethysmography and all healthy subjects underwent spirometry. A time-series of one coronal slice centered to the trachea was acquired for 60 s of RTB and 30 s of MPT at 1.5 T (MAGNETOM Avanto, Siemens Healthineers, Erlangen, Germany) as part of a phase-resolved functional lung (PREFUL)-MRI4 acquisition. A 2D SPGR sequence was used (see Table 1a for details). The whole procedure was performed twice. For every slice of the time-series the lung area was segmented using a state-of-the-art U-Net.5 Fractional ventilation (FV) was calculated for RTB and MPT according to equation (1)4,6$$ FV= \frac{(A_{inspiration}-A_{expiration})}{A_{inspiration}} (1)$$
where A_ represents the lung area in inspiration/expiration.
Further, in order to assess dynamic hyperinflation1, the end-expiratory lung area (EELA) of the coronal image slice was measured at the beginning and end of MPT by calculating the mean lung area of the end-expiratory states during the first and last five seconds of MPT, respectively. In order to account for differences in height and weight, the EELA was body surface area normalized. Ratios of FV between MPT and RTB and ratios of EELA between the end and start of MPT were formed and compared between both repetitions for each study group. Paired t-tests with Bonferroni correction for multiple comparisons (α=0.025) and Bland-Altman analyses were applied. Furthermore, ratios of both ventilation parameters were compared between the three study groups. Unpaired t-tests were used to compare the young-healthy with older-healthy group as well as the older-healthy with the COPD group. A Bonferroni correction for multiple comparisons was applied (α=0.025).
Results
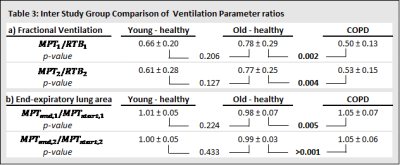
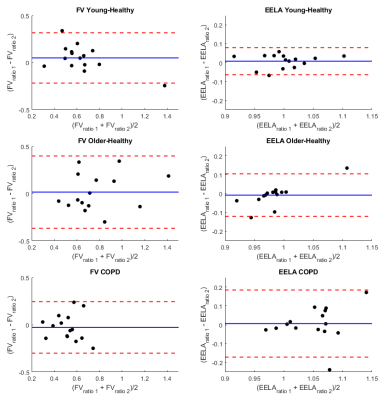
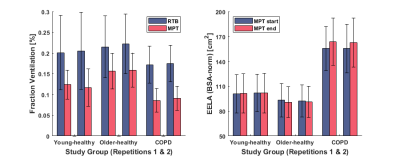
The age- and sex-distribution as well as body plethysmography / spirometry parameters of the cohort are shown in Table 1b. The results of repeatability analysis of FV and EELA ratios for each study group are shown in Table 2. Bland-Altman plots for both ventilation parameters and each study group are shown in Figure 1. No significant differences were found between both repetitions of the procedure. Comparisons of the ventilation parameters between study groups shown in Table 3 yielded no significant differences between both healthy groups. However, COPD patients had a significantly larger decrease in FV than older-healthy subjects and a significant increase in EELA in both repetitions of the procedure. Figure 2 visualizes the mean differences of FV between RTB and MPT and the differences of EELA at the start and end of MPT as well as the respective standard deviations.Discussion
MPT induces dynamic hyperinflation in COPD patients and has a significant effect on ventilation dynamics as they suffer primarily from expiratory airflow obstruction.1,7 In this study, COPD patients were shown to be significantly affected by MPT as demonstrated by changes in FV and EELA. Even though FV of healthy subjects was affected by MPT, the decrease in FV during MPT was significantly higher in COPD patients compared to age and sex matched healthy subjects. No significant difference between the two healthy groups was observed, indicating that MPT has a stronger influence on FV in COPD patients than in healthy subjects. The examination of EELA in MPT yielded similar results. The increase in EELA, observed only in COPD patients, is likely due to dynamic hyperinflation.3 Both parameters have been shown to be repeatable over the two repetitions. The results agree with previous studies that have shown that MPT leads to dynamic hyperinflation and is a valid surrogate for exercise testing.3,8,9 Even though repeatability has been shown, it is necessary to investigate the reproducibility of the two proposed parameters with data from different cohorts and study centers.Conclusion
MPT-induced global ventilation changes are reliably detected with MR imaging using a single coronal slice at the level of the trachea. Expiratory lung area ratio and global fractional ventilation ratio are promising sensitive MR imaging parameters to examine MPT-induced hyperinflation in COPD patients.Acknowledgements
No acknowledgement found.References
1. O'Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 2001;164(5):770-7.
2. Klooster K, ten Hacken NH, Hartman JE, Sciurba FC, Kerstjens HA, Slebos DJ. Determining the role of dynamic hyperinflation in patients with severe chronic obstructive pulmonary disease. Respiration. 2015;90(4):306-13.
3. Weigt SS, Abrazado M, Kleerup EC, Tashkin DP, Cooper CB. Time course and degree of hyperinflation with metronome-paced tachypnea in COPD patients. COPD: Journal of Chronic Obstructive Pulmonary Disease. 2008;5(5):298-304.
4. Voskrebenzev A, Gutberlet M, Becker L, Wacker F, Vogel-Claussen J. Reproducibility of fractional ventilation derived by Fourier decomposition after adjusting for tidal volume with and without an MRI compatible spirometer. Magn Reson Med 2016;76:1542–1550.
5. Ronneberger O, Fischer P, Brox T. U-net: Convolutional networks for biomedical image segmentation. International Conference on Medical image computing and computer-assisted intervention 2015;234:241.
6. Zapke M, Topf H-G, Zenker M, Kuth R, Deimling M, Kreisler P, Rauh M, Chefd’hotel C, Geiger B, Rupprecht T. Magnetic resonance lung function—a breakthrough for lung imaging and functional assessment? A phantom study and clinical trial. Respir Res 2006;7:106.
7. Cooper CB, Calligaro GL, Quinn MM, Eshaghian P, Coskun F, Abrazado M, Bateman ED, Raine RI. Determinants of dynamic hyperinflation during metronome-paced tachypnea in COPD and normal subjects. Respiratory physiology & neurobiology. 2014;190:76-80.
8. Gelb AF, Gutierrez CA, Weisman IM, Newsom R, Taylor CF, Zamel N. Simplified detection of dynamic hyperinflation. Chest. 2004 Dec; 126(6): 1855–60.
9. Calligaro GL, Raine RI, Bateman ME, Bateman ED, Cooper CB. Comparing dynamic hyperinflation and associated dyspnea induced by metronome-paced tachypnea versus incremental exercise. COPD. 2014 Feb; 11(1): 105–12.
Figures