4664
129Xe MRI Assessment of Disease Progression in Patients with COPD treated with Azithromycin: Multi-Site Repeatability and Preliminary Results1University of Virginia, Charlottesville, VA, United States, 2Duke University, Durham, NC, United States, 3Genentech, San Francisco, CA, United States, 4University of Kansas, Lawrence, KS, United States
Synopsis
Keywords: Lung, Hyperpolarized MR (Gas), COPD, clinical trial
Harmonization of imaging methodology among three sites was successfully achieved.
Preliminary results for longitudinal treatment with azithromycin demonstrated the sensitivity of 129Xe MRI in detecting ventilation changes, presumably caused by Sars-Cov2 infection or termination of azithromycin treatment at week 24 due to hearing loss. This preliminary data supports that 129Xe MRI is a sensitive tool for detecting subtle changes in COPD early and may potentially reduce the length of clinical trials that aim to visualize therapeutic responses.
Introduction
Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) occur unpredictably and require significant healthcare resources. Currently available biomarkers are incapable of predicting the efficacy of the treatment regimens to prevent/reduce AECOPD. A detailed phenotyping of AECOPD is necessary to understand its natural history and to effectively select the most appropriate treatment. MRI using hyperpolarized xenon-129 gas (129Xe) is an emerging technology capable of providing, in a single breath-hold, detailed ventilation images of the lungs as well as physiological maps of 129Xe distribution into multiple lung compartments (gas-exchange), specifically into lung parenchyma (barrier/membrane) and red-blood-cells (RBC)1,2,3. Our recent study using 129Xe MRI in patients with COPD shows the complex pulmonary pathophysiology that can influence COPD patients’ therapeutic responses and natural history of AECOPD4. Furthermore, 129Xe MRI studies in healthy subjects and “healthy smokers” discriminated these two groups before the spirometry detected loss of lung function5, suggesting that 129Xe MRI is a sensitive tool for detecting early-stage lung disease. These reports highlight the potential of 129Xe MRI to significantly reduce the length of clinical trials that aim to address AECOPD.Methods and Materials
Multi-site repeatability:This is the first multi-site clinical trial using 129Xe MRI for both ventilation and gas-exchange imaging to probe multiple lung compartments (ventilation, barrier/membrane and RBC). Its first aim is to assess harmonization and repeatability of 129Xe imaging methodology among sites, on different MRI scanner platforms, particularly for the gas-exchange technique. The one-point Dixon technique1 was selected for gas-exchange measurements along with the post-processing software package developed by Duke University. To qualify for participation each of three sites (University of Virginia, Duke University and University of Kansas) recruited one healthy volunteer to undergo repeat 129Xe MRI studies (all females; age 34.7±6.1). The pulse sequence protocols used for this study were previously described in detail6 and adopted by the 129Xe MRI Clinical Trial Consortium. A 2D-GRE pulse sequence was used for ventilation acquisitions and a one-point Dixon pulse sequence for gas-exchange, which were repeated on the same subject within 8 hours. Each site polarized 129Xe using a commercial polarizer (model Xe9820, Polarean Inc, USA), achieving polarizations of 25-30% and employed identical versions of the same commercially available RF vest-coil, tuned to the 129Xe frequency at 3 Tesla (Clinical MR Solutions, USA). The total volume of 129Xe gas mixed with nitrogen was approximately 20% of the subject’s FVC, measured by spirometry prior to the MRI scan.
Longitudinal treatment with azithromycin:
The clinical trial aims to assess longitudinal changes in ventilation defect percentage (VDP), barrier defect percentage (BDP) and RBC defect percentage (RDP) over a 48-week period. These results will be analyzed to determine if the metrics can predict rates of exacerbation or clinical benefit from daily treatment with azithromycin. The study design is shown in Figure 1. In addition to 129Xe MRI, subjects also received full pulmonary function tests (PFT) and high-resolution computed tomography (HRCT). The overall goal is to enroll 120 subjects (cohort A:100 GOLD 2-4; cohort B:20 GOLD 1) across 7 sites. To date, one subject has completed the 48-week assessment. We report preliminary results from this subject (66 YO; male; 22.5 pk/yr smoker; history of COPD, Covid19 infection 12 months prior to study and re-infection at week 14 during study), who had to stop azithromycin treatment at week 24 due to hearing loss.
Results
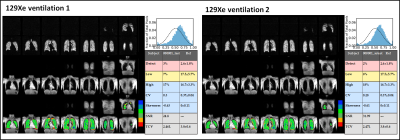
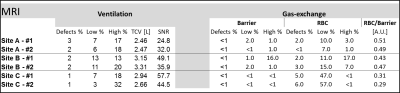
Multi-site repeatability:Figures 2 and 3 show all images, with detailed global analyses, for the repeatability scans from a representative subject. Note the repeatable, almost identical, ventilation (Fig. 2) and gas-exchange (Fig. 3) images. Quantitative analysis for each 129Xe MRI scan for all sites is presented in Figure 4 and corroborates the highly repeatable results obtained.
Longitudinal treatment with azithromycin:
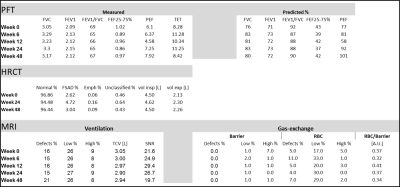
Results for PFT, HRCT and 129Xe MRI ventilation and gas-exchange, for each of the time-points, are presented in Figure 5. PFT and HRCT did not show statistically significant changes from baseline (week 0) to week 48, for any of the parameters assessed. 129Xe MRI ventilation showed a statistically significant difference for the percentage of ventilation defects at week 48 (VDP=21%) compared with earlier time-points (VDP=15.5±0.5%). RBC defect percentage (RDP) at week 6 was also significantly larger (RDP=11%) than that at all other time points (RDP=5.25±1.09%).
Discussion & Conclusion
Harmonization of imaging methodology among three sites was successfully achieved, opening the way for the clinical trial with 120 COPD patients. Intra-site repeatability was good, although there were differences between sites.Preliminary results for longitudinal treatment with azithromycin demonstrated the sensitivity of 129Xe MRI in detecting ventilation changes, presumably caused by Sars-Cov2 infection or termination of azithromycin treatment at week 24 due to hearing loss. At week 48, the subject reported slight worsening of dyspnea, although PFT and HRCT were not able to detect significant changes while significant changes were detected using 129Xe MRI. This preliminary data supports that 129Xe MRI is a sensitive tool for detecting subtle changes in COPD early and may potentially reduce the length of clinical trials that aim to visualize therapeutic responses.
Acknowledgements
Study supported by Genentech Inc.References
1- Driehuys B, Martinez-Jimenez S, Cleveland Z, et al. Safety and tolerability of hyperpolarized 129Xe MR imaging in healthy volunteers and patients. Radiology 2012;262:279-89.
2- Joseph G. Mammarappallil, EM, et al. Identification of gas exchange phenotypes using hyperpolarized 129Xe MRI in patients with chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med 2019; 199:A1122.
3- Guan S, Tustison N, Qing K, et al. 3D Single-Breath Chemical Shift Imaging Hyperpolarized Xe-129 MRI of Healthy, CF, IPF, and COPD Subjects. Tomography 2022; 8(5):2574-2587.
4- Myc L, Qing K, He M, et al. Characterization of gas exchange in COPD with dissolved-phase hyperpolarised xenon-129 MRI. Thorax 2021;76:178-181.
5- Qing K, Tustison NJ, Mugler JP III, et al. Probing changes in lung physiology in COPD using CT, perfusion MRI, and hyperpolarized Xenon-129 MRI. Acad Radiol 2019; 26:326-34.
6- Niedbalski PJ, Hall CS, Castro M, et al. Protocols for multi-site trials using hyperpolarized 129Xe MRI for imaging of ventilation, alveolar-airspace size, and gas exchange: A position paper from the 129Xe MRI clinical trials consortium. Magn Reson Med 2021; 86:2966-2986.
Figures