4647
Accelerated MRI near metallic implants at 0.55T using hexagonal sampling
Bahadir Alp Barlas1, Kubra Keskin1, Brian A Hargreaves2,3,4, and Krishna S Nayak1,5
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
Synopsis
Keywords: Data Acquisition, Low-Field MRI
Metallic implants cause severe distortions in the magnetic field that are best mitigated using multi-spectral acquisitions that require longer scan time. At conventional field strengths, this is compensated by aggressive use of parallel imaging. However, at low-field strengths (<1.5T), available parallel imaging factors are reduced because body noise dominance prevents the use of dense arrays with smaller elements. Hexagonal sampling (in ky,kz space) was previously proposed as a way to achieve 2-fold reduction in imaging time without introducing additional artifacts. In this work, we demonstrate applicability of the hexagonal sampling for 0.55T multi-spectral imaging near metal.INTRODUCTION
Metallic implants distort the static magnetic field, leading to severe artifacts when using conventional MRI pulse sequences1,2. Multi-spectral imaging techniques such as MAVRIC, SEMAC, and MAVRIC-SL, effectively eliminate these artifacts by encoding an additional dimension, either directly encoding frequency with a non-selective excitation approach (MAVRIC) or indirectly encoding frequency by resolving slice-direction distortion (SEMAC or MAVRIC-SL)3,4. This requires longer scan times, typically compensated for by using parallel imaging and partial Fourier acquisitions5.New low-field MRI systems show great potential for improved imaging adjacent to metallic implants6,7. However, at lower field strengths (<1.5T)8,9, it is more challenging to produce dense RF coil arrays with small elements, without compromising body noise dominance10. This makes it challenging to achieve high parallel imaging factors.
Hexagonal sampling (in ky,kz space) has been proposed to achieve 50% scan time reduction without introducing additional artifacts at 1.5T11. In this work, we demonstrate the effectiveness of SEMAC with hexagonal sampling at 0.55T in phantoms and in vivo.
METHODS
Experimental Methods: Experiments were performed on a 0.55T whole-body system (prototype MAGNETOM Aera, Siemens Healthineers, Erlangen, Germany) equipped with high-performance shielded gradients (45 mT/m amplitude, 200 T/m/s slew rate).Phantom Experiment: A fully-sampled SEMAC scan of a hip implant with a titanium stem and a cobalt chromium head was acquired with parameters: 300x243.75 mm2 FOV, 4 mm slice thickness, 320x260 base resolution, 20 slices, SEMAC factor 12.
Volunteer Experiment: One healthy volunteer with spinal vertebral body tethering system was scanned in a supine position after providing written informed consent and under a protocol approved by our Institutional Review Board. Data were collected using 6-elements of the table-integrated spine array (posterior) and a 6-channel body coil (anterior). Sagittal SEMAC scan parameters were: 279x279 mm2 FOV, 4 mm slice thickness, 320x320 base resolution, 21 slices, SEMAC factor 12.
Analysis: As shown in Figure 1, fully-sampled ky,kz data (black dots) were retrospectively subsampled in a checkerboard pattern (black dots sampled, gray dots discarded), simulating 50% decrease in the acquisition time. This causes the aliased replicas to appear in the diagonal direction in y-f space. It is assumed that the metallic implant is approximately centered in the FOV along the y-direction, and that the width of the implant is smaller than FOVy/2, ensuring minimal overlap between the main profile and aliased replicas. Masks, are then applied in y,f space to zero-out these replicas. The design of the two masks prioritizes robust elimination of the aliased profiles while maintaining the main lobe signal. The appropriateness of the masks was tested by comparing the energy levels of the masked data with the fully sampled data. Then, the Sum-of-Squared (SOS) reconstruction of SEMAC images for each mask were obtained from the undersampled data from the phantom and volunteer experiments. Lastly, image noise levels and its dependence on y-position was computed from z,x-positions outside of the phantom, to quantify the spatially varying noise due to masking.
RESULTS
Figure 2 shows the central slice from a SEMAC scan of a hip implant with several representative y-f signal profiles. These show that the cobalt chromium head significantly distorts the profiles while the titanium stem has less effect.Figure 3 illustrates SEMAC reconstruction for fully sampled and hexagonally undersampled cases with both masks. By visual inspection, image quality with both Mask 1 and Mask 2 are comparable to the fully sampled case. However, considerable SNR reduction can be observed, especially for the Mask 1 case due to its sharp profile. The noise variance as a function of y-position is shown, and matches the y,f mask shape.
Figure 4 illustrates the y-f signal profiles for fully sampled and hexagonally undersampled cases with both masks. Both masks successfully remove most of the aliased signal. Mask 1 suffers slightly from residual aliasing (yellow arrows). Mask 2 provides robust aliasing suppression, but also slight signal loss of the main profile for extremely distorted profiles (white arrow).
Figure 5 shows in-vivo sagittal SEMAC images where metallic distortion is significant. The image for Mask 1 suffers from FOVy/2 aliasing artifacts due to the unsuppressed replica signal similar to yellow arrows in Figure 4. The image for Mask 2 achieves similar image quality compared to the fully sampled case.
DISCUSSION
We demonstrate SEMAC with hexagonal sampling at 0.55T. This approach provides rate-2 acceleration, and is advantageous at low field strengths where high parallel imaging factors are challenging. Further optimization of the mask is possible. For instance, slightly smaller masks can be combined with complementary hexagonal sampling12.CONCLUSION
Hexagonal sampling shortens scan time by 50% in 0.55T SEMAC, and introduces some mild spatial variation in SNR. Reconstructed image quality is comparable to the fully sampled case. This may be appropriate in scenarios where the applicability of parallel imaging acceleration is limited.Acknowledgements
We acknowledge grant support from the National Institutes of Health (R01-AR078912), and research support from Siemens Healthineers. We thank Sophia Cui for her help with the SEMAC protocols, and Mary Yung for research coordination.References
- Hargreaves, B. A., Worters, P. W., Pauly, K. B., Pauly, J. M., Koch, K. M., & Gold, G. E. (2011). Metal-Induced Artifacts in MRI. American Journal of Roentgenology, 197(3), 547–555. https://doi.org/10.2214/AJR.11.7364
- Dillenseger, J. P., Molière, S., Choquet, P., Goetz, C., Ehlinger, M., & Bierry, G. (2016). An illustrative review to understand and manage metal-induced artifacts in musculoskeletal MRI: a primer and updates. Skeletal Radiology, 45(5), 677–688. https://doi.org/10.1007/s00256-016-2338-2
- Koch, K. M., Hargreaves, B. A., Pauly, K. B., Chen, W., Gold, G. E., & King, K. F. (2010). Magnetic resonance imaging near metal implants. Journal of Magnetic Resonance Imaging, 32(4), 773–787. https://doi.org/10.1002/jmri.22313
- Jungmann, P. M., Agten, C. A., Pfirrmann, C. W., & Sutter, R. (2017). Advances in MRI around metal. Journal of Magnetic Resonance Imaging, 46(4), 972–991. https://doi.org/10.1002/jmri.25708
- Hargreaves, B. A., Chen, W., Lu, W., Alley, M. T., Gold, G. E., Brau, A. C. S., Pauly, J. M., & Pauly, K. B. (2010). Accelerated slice encoding for metal artifact correction. Journal of Magnetic Resonance Imaging, 31(4), 987–996. https://doi.org/10.1002/jmri.22112
- Khodarahmi, I., Brinkmann, I. M., Lin, D. J., Bruno, M., Johnson, P. M., Knoll, F., Keerthivasan, M. B., Chandarana, H., & Fritz, J. (2022). New-Generation Low-Field Magnetic Resonance Imaging of Hip Arthroplasty Implants Using Slice Encoding for Metal Artifact Correction. Investigative Radiology, 57(8), 517–526. https://doi.org/10.1097/RLI.0000000000000866
- Khodarahmi, I., Keerthivasan, M.B., Brinkmann, I.M., Grodzki, D. and Fritz, J., 2022. Modern Low-Field MRI of the Musculoskeletal System: Practice Considerations, Opportunities, and Challenges. Investigative Radiology, pp.10-1097. https://doi.org/10.1097/RLI.0000000000000912
- Marques, J. P., Simonis, F. F. J., & Webb, A. G. (2019). Low‐field MRI: An MR physics perspective. Journal of Magnetic Resonance Imaging, 49(6), 1528–1542. https://doi.org/10.1002/jmri.26637
- Campbell-Washburn, A. E., Ramasawmy, R., Restivo, M. C., Bhattacharya, I., Basar, B., Herzka, D. A., Hansen, M. S., Rogers, T., Bandettini, W. P., McGuirt, D. R., Mancini, C., Grodzki, D., Schneider, R., Majeed, W., Bhat, H., Xue, H., Moss, J., Malayeri, A. A., Jones, E. C., … Balaban, R. S. (2019). Opportunities in Interventional and Diagnostic Imaging by Using High-Performance Low-Field-Strength MRI. Radiology, 293(2), 384–393. https://doi.org/10.1148/radiol.2019190452
- Gruber, B., Froeling, M., Leiner, T., & Klomp, D. W. J. (2018). RF coils: A practical guide for nonphysicists. Journal of Magnetic Resonance Imaging, 48(3), 590–604. https://doi.org/10.1002/jmri.26187
- Sveinsson, B., Worters, P. W., Gold, G. E., & Hargreaves, B. A. (2015). Hexagonal undersampling for faster MRI near metallic implants. Magnetic Resonance in Medicine, 73(2), 662–668. https://doi.org/10.1
- Sveinsson, B., Taviani, V., Gold, G., & Hargreaves, B. (2015). Automatic Detection of Metal Implant Location in Hexagonally Sampled MAVRIC-SL. In Proc. Intl. Soc. Mag. Reson. Med (Vol. 23, p. 2508).
Figures
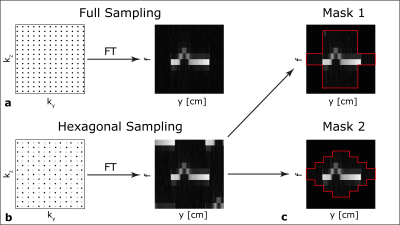
Figure 1: Overview of hexagonal sampling and masking. (a) Fully-sampled k-space and its corresponding data. (b) Hexagonal undersampling positions the aliased replicas lie along the diagonal direction due to the checkerboard pattern undersampling (black dots sampled, gray dots discarded). (c) Mask 1 and Mask 2 are designed to suppress the aliased replicas while preserving the main profile. Aliased replicas are removed from a distorted profile with Mask 1 and Mask 2 while preserving the main profile.
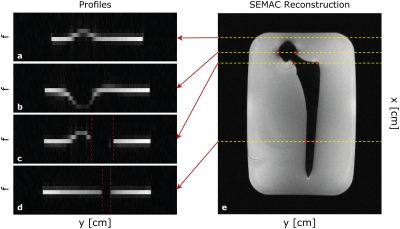
Figure 2: The SEMAC reconstructed image for a central slice together with segments in the y-f plane corresponding to the shown x-positions (yellow dashed lines), with x as up-down in this image, y across and f through-plane. Profiles show that the cobalt chromium head significantly distorts the profiles across f-direction while the titanium stem has no effect on the profiles (red dashed lines). The fashion of the distorted regions are consistent with the masks provided in Figure 1.
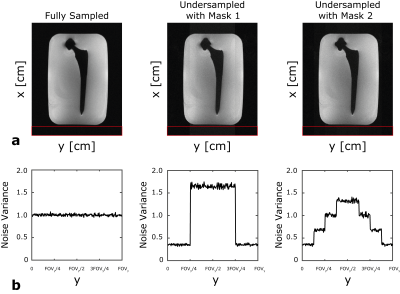
Figure 3: (a) Sum-of-Squared (SOS) reconstruction of SEMAC images of a central slice. The quality of metal artifact correction of both Mask 1 and Mask 2 are comparable to the fully sampled case, while the reduced SNR is visible where the mask amplitude is higher, especially for Mask 1. (b) Noise variance is computed using the area denoted by the red frame as a function of y-position. The design of the masks directly determined the SNR levels of the reconstructed images.

Figure 4: Simulated profiles in the y-f plane. Subfigures represent the segments for the fully sampled, hexagonal, hexagonal followed by Mask 1 and Mask 2 (red frames). Mask 1 introduced some aliasing signals in the edges of the mask (yellow arrows). Although the profiles for Mask 2 show significant aliasing suppression, (b) they may suffer from signal loss of the main profile (white arrow).
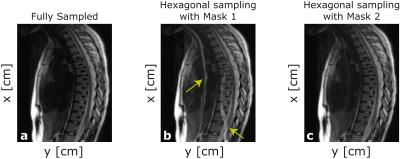
Figure 5: Sum-of-Squared reconstruction of SEMAC images acquired from the volunteer experiment for the cases of (a) fully sampled, undersampling followed by (b) Mask 1 and (c) Mask 2. Image quality of (b) Mask 2 case is comparable to the fully sampled case, while the image for Mask 1 suffers from aliasing artifacts (yellow arrows). As in the case shown in Figure 4, since Mask 1 allows central FOVy/2 even for distant SEMAC values, a portion of replica signal remains unsuppressed, causing aliasing artifacts.
DOI: https://doi.org/10.58530/2023/4647