4635
Fat/water separated & SMS accelerated pseudo 3D PROPELLER1Karolinska Instituet, Stockholm, Sweden, 2Karolinska University Hospital, Stockholm, Sweden, 3MR Applied Science Laboratory Europe, GE Healthcare, Munich, Germany, 4Uppsala University Hospital, Uppsala, Sweden
Synopsis
Keywords: Data Acquisition, Data Acquisition, MSK, Dixon, SMS
By combining thin slices, PROPELLER acquisition, SMS acceleration and chemical shift encoding with asymmetrical gradients reformattable pseudo 3D volumes can be acquired. These volumes are, in contrast to 3D RARE volumes, efficiently chemical shift encoded, less susceptible to T2-blurring and less sensitive to motion.
Introduction
High resolution 3D MRI data that is reformattable in any plane is often preferred in the clinical routine. 3D RARE sequences are a common choice for acquiring these data sets. However, the very long echo trains of 3D RARE sequences cause image blurring, motion sensitivity and forces these sequences to use very short echo spacings typically around 4 ms (1). In this very short time frame it is challenging to achieve the chemical shift encoding needed for fat/water separation. Fat/water separation also has high clinical value, especially in musculoskeletal (MSK) imaging, and also benefits from being reformattable in any plane.It has been shown that, with SMS acceleration, it is possible to rapidly obtain thin-sliced Cartesian 2D (“pseudo 3D”) data that may be reformatted in other planes (2). We have previously shown that acquiring pseudo 3D volumes with a SMS accelerated PROPELLER sequence allows for flow and motion robust imaging (3). We have also shown that chemical shift encoding can be efficiently achieved in 2D RARE with asymmetric readout gradients (4).
In this work we combine the SMS accelerated pseudo 3D PROPELLER acquisition with chemical shift encoding using asymmetric readout gradients, to acquire reformattable fat/water separated image volumes.
Methods
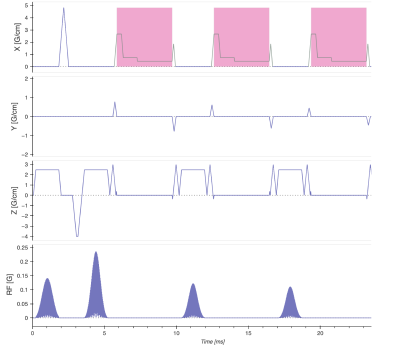
The pseudo 3D PROPELLER data set was acquired with 1 mm3 voxels, i.e. the slice thickness was 1 mm. An intermediate-TE PD-weighting (5) was achieved with a TE of 40 ms and TR of 7500 ms. To produce chemical shift encoding a multi-TR asymmetric readout waveforms approach was used. In a multi-TR fat/water separated PROPELLER sequence the chemical shift encoding is realized by acquiring the same blade twice, first with in-phase echoes and then with out-of-phase echoes one TR later. The in-phase echoes were acquired with a regular constant amplitude readout gradient and the out-of-phase echoes were acquired with an asymmetric readout gradient (Figure 1). An in-plane acceleration factor of 2 was combined with an SMS acceleration factor of 2, amounting to a total scan time of 3:24 min. A volunteer was scanned using a 3T system (GE Healthcare, Milwaukee, WI, USA) with a Tx/Rx 16 channel knee coil. The pulse sequence was programmed using the “KSFoundation” abstraction layer for EPIC (6). Parallel imaging reconstruction was performed on the echos separately before the fat/water separation using the respective methods described in references: (3,4).Results
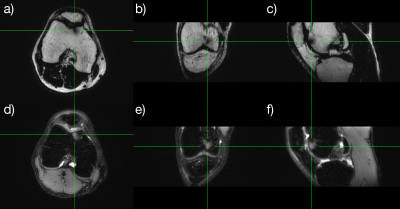
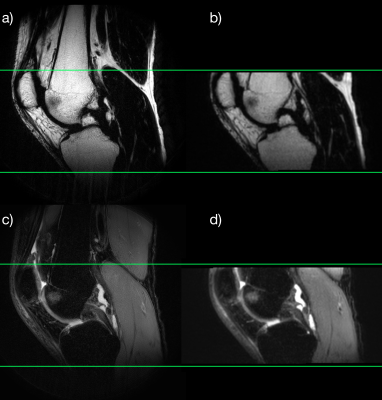
Resulting images (Figure 2) show that high quality reformattable fat/water separated volumes can be acquired with an SMS accelerated PROPELLER sequence in a reasonable time frame. A small area of subchondral bone marrow edema of the volunteer was clearly visualized in all three planes. Figure 3 compares a sagittal pseudo 3D reformat to a high resolution image acquired in the sagittal plane, showing the reformat quality achievable with 1 mm slices.Discussion
The acquisition of pseudo 3D fat/water separated images using the SMS accelerated PROPELLER sequence has several advantages when compared to a 3D RARE acquisition. Namely: more freedom to efficiently encode the chemical shift, less T2-blurring and reduced sensitivity to motion.In this work the sequence was used to acquire intermediate-TE PD-weighted MSK images. However the method is generally applicable to any contrast that can be acquired with a 2D RARE sequence.
Since the fat signal is excited at a slightly different slice position, due to the chemical shift, it will experience a different CAIPIRINHA shift, which would cause ghosts. This could easily be corrected for in the reconstruction. However, the very thin slices (high slice selective gradient strength) seem to have made the effect negligible so it could be ignored in this particular case.
Acknowledgements
No acknowledgement found.References
1. Mugler JP 3rd. Optimized three-dimensional fast-spin-echo MRI. J. Magn. Reson. Imaging 2014;39:745–767 doi: 10.1002/jmri.24542.
2. Gagoski BA, Bilgic B, Eichner C, et al. RARE/turbo spin echo imaging with Simultaneous Multislice Wave-CAIPI. Magn. Reson. Med. 2015;73:929–938 doi: 10.1002/mrm.25615.
3. Norbeck O, Avventi E, Engström M, Rydén H, Skare S. Simultaneous multi-slice combined with PROPELLER. Magn. Reson. Med. 2018;80:496–506 doi: 10.1002/mrm.27041.
4. Rydén H, Norbeck O, Avventi E, et al. Chemical shift encoding using asymmetric readout waveforms. Magn. Reson. Med. 2021;85:1468–1480 doi: 10.1002/mrm.28529.
5. Crema MD, Roemer FW, Marra MD, et al. Articular cartilage in the knee: current MR imaging techniques and applications in clinical practice and research. Radiographics 2011;31:37–61 doi: 10.1148/rg.311105084.
6. Skare S, Avventi E, Norbeck O, Ryden H. An abstraction layer for simpler EPIC pulse programming on GE MR systems in a clinical environment. In: Proc. Intl. Soc. Mag. Reson. Med. 25. ; 2017. p. 3813.
Figures