4606
Distinguish Injured from Intact Axons in Coherent Fiber Bundles Using Network Estimation Operator (NEO)1Radiology, Washington University School of Medicine in Saint Louis, Saint Louis, MO, United States, 2Department of Mathematics and Statistics, Washington University in Saint Louis, Saint Louis, MO, United States
Synopsis
Keywords: Signal Modeling, Diffusion Tensor Imaging
Deep Neural Network based Network Estimation Operator (NEO) was developed to differentiate and quantify parallel fibers of different axial diffusivities. Data from both Monte Carlo simulations and an In-Silico imaging phantom were analyzed by both DBSI and NEO. Results reveal that NEO distinguished parallel fibers of different axial diffusivity while DBSI only identified a single fiber. NEO can detect and quantify injured vs. non-injured axons in a coherent fiber bundle. Thus, NEO can quantify the extent of axonal injury and loss more accurately than DBSI and other advanced diffusion MRI models.Introduction
Multi-tensor models represent a direct extension of conventional DTI, providing additional microstructural information from the estimation of several diffusion tensors. However, given the non-convex solution space and number of free variables associated with multi-tensor estimators, there is an unmet need for a fast, accurate, and flexible framework for multi-tensor estimation.We propose that Deep Neural Networks (DNNs) can be leveraged to be such a tool. Data-driven learning already shows promise for estimating fiber ODF’s.1 We adapt DNNs to the multi-diffusion-tensor estimation problem by enforcing a network architecture such that the diffusion tensors and volume fractions of interest are the weights of the network. This way, we can leverage DNNs to create a framework for general diffusion-weighted MRI (dMRI) signal modeling and tensor estimation which has access to future advancements in machine learning and the optimization power of back-propagation enabled gradient methods such as Adam.2
In this abstract, we will demonstrate the effectiveness of a DNN based Network Estimation Operator (NEO) in delineating parallel fibers of different diffusivities.
Methods
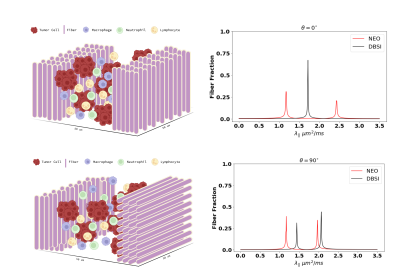
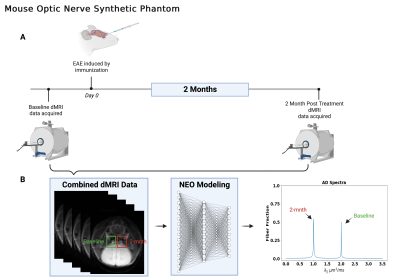
Monte-Carlo (MC) Simulation: Intra-voxel crossing-fiber (0 and 90°) configurations were developed for MC simulation. The simulation was performed by allowing water molecules (2.25 × 105, uniformly distributed) to undergo a random walk within a 50-μm cubic voxel (Fig. 1). Free water diffusivity = 3 $$$\mu m^{2}/ms$$$ and intra-fiber axial diffusivity = 1 and 2 $$$\mu m^{2}/ms$$$ at 30 °C. The time-step for the random walk was 0.001 $$$ms$$$.In-Silico Phantom: An imaging phantom consisting of two parallel fiber tracts with different axial diffusivities was constructed by combining in vivo dMRI optic nerve signals of an Experimental Autoimmune Encephalomyelitis (EAE) mouse (Fig. 2). Healthy control (HC) and EAE optic nerve data were simulated by obtaining dMRI signals from the same mouse at 2 weeks before and 2 months after EAE induction. Detailed experimental procedures were reported previously.3 Manually defined ROIs were applied to the left and right optic nerves, and the corresponding signals were averaged and then analyzed by both DBSI4 and NEO to estimate the axial diffusivity (AD, $$$\lambda_{||}$$$) in the HC and EAE optic nerves respectively. The baseline and 2-month dMRI signals were combined by joining the EAE signal to the HC signal (HC + EAE). The combined (HC + EAE) signal was analyzed by DBSI and NEO to evaluate the ability of the diffusion MRI models to recover the HC and EAE $$$\lambda_{||}$$$ from single voxel data (Fig. 2).
Results
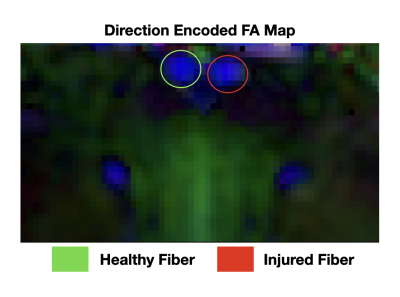
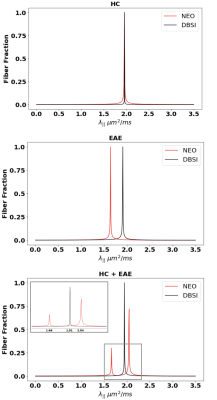
Monte-Carlo Simulation: For combined dMRI signals, DBSI estimated a single $$$\lambda_{||}$$$ = 1.71 $$$\mu m^{2}/ms$$$ for the parallel configuration, and $$$\lambda_{||}$$$ = 1.43 and 2.05 $$$\mu m^{2}/ms$$$ respectively for the perpendicular configurations (Fig. 1). In contrast, NEO estimated $$$\lambda_{||}$$$ = 1.17 and 2.4 $$$\mu m^{2}/ms$$$ for parallel and $$$\lambda_{||}$$$= 1.1 and 1.96 $$$\mu m^{2}/ms$$$ for the perpendicular condition (Fig. 1).In-Silico Phantom: Direction encoded fractional anisotropy (FA) plotting of the dMRI results confirmed that the examined optic nerves were indeed parallel to each other (Fig. 3). For HC optic nerves, NEO and DBSI estimated $$$\lambda_{||}$$$= 1.948 and 1.951 $$$\mu m^{2}/ms$$$, respectively. NEO estimated EAE optic nerve $$$\lambda_{||}$$$ = 1.635 μm 2/ms while DBSI returned an estimate of 1.901 $$$\mu m^{2}/ms$$$. On the combined HC + EAE optic nerves, NEO estimated $$$\lambda_{||}$$$ =1.657 and 2.037 $$$\mu m^{2}/ms$$$ with fiber fractions = 0.30 and 0.70 respectively (Fig. 4). NEO’s error with respect to the “ground truth $$$\lambda_{||}$$$” obtained from the HC and EAE signal alone are 4.57% and 1.34% respectively. DBSI found only one $$$\lambda_{||}$$$= 1.939 $$$\mu m^{2}/ms$$$ for HC + EAE data (Fig. 4).
Discussion
Results demonstrate that DBSI did not delineate parallel fibers of different $$$\lambda_{||}$$$ while NEO identified and quantified $$$\lambda_{||}$$$ and fiber fractions of the parallel fiber tracts. The DNN-based NEO is thus a usable tool to detect and differentiate injured from non-injured axons in a coherent fiber tract. This platform could be applied in clinical and research settings to more accurately quantify the extent of axonal injury/loss in nerve fiber tracts in patients with various neurodegenerative diseases.Acknowledgements
No acknowledgement found.References
1. Davood Karimi, Lana Vasung, Camilo Jaimes, Fedel Machado-Rivas, Simon K. Warfield, Ali Gholipour,Learning to estimate the fiber orientation distribution function from diffusion-weighted MRI, NeuroImage, Volume 239, 2021, 118316, ISSN 1053-81192.
2. Diederik P. Kingma Jimmy Ba. 2015 conf/iclr/2015 ICLR (Poster)
3. Lin TH, Zhan J, Song C, Wallendorf M, Sun P, Niu X, Yang R, Cross AH, Song SK. Diffusion Basis Spectrum Imaging Detects Axonal Loss After Transient Dexamethasone Treatment in Optic Neuritis Mice. Front Neurosci. 2021 Jan 22;14:592063. doi: 10.3389/fnins.2020.592063. PMID: 33551721; PMCID: PMC7862582.
4. Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain 2011;134:3590–3601.
Figures