4601
Impact of free-water correction on white matter changes measured by diffusion tensor imaging in migraine1Institute for Systems and Robotics - Lisboa and Department of Bioengineering, Instituto Superior Técnico, Universidade de Lisboa, Lisbon, Portugal, 2Universidad Autónoma de Madrid, Madrid, Spain, 3Laboratorio de Procesado de Imagen, Universidad de Valladolid, Valladolid, Spain, 4Learning Health, Hospital da Luz, Lisbon, Portugal, 5Imaging Department, Hospital da Luz, Lisbon, Portugal, 6Neurology Department, Hospital da Luz, Lisbon, Portugal, 7Center for Interdisciplinary Research in Health, Universidade Católica Portuguesa, Lisbon, Portugal
Synopsis
Keywords: Signal Modeling, Diffusion/other diffusion imaging techniques
Menstrual migraine affects about 25% of female migraine patients. However, the diagnosis of migraine is particularly difficult because the brain changes associated with migraine are challenging to detect with imaging techniques. Diffusion-weighted MRI (dMRI) permits the detection of alterations in the microenvironment of the brain tissues. We investigate whether removing the contribution of the free water component from the diffusion-signal can provide increased sensitivity to identify white matter changes in migraine using diffusion tensor metrics.Introduction
Migraine is one of the most common neuropathologies in the world affecting about 17% of people, and the most disabling neurological disorder1. Among the different types of migraine, menstrual migraine affects nearly a quarter of female migraine patients2. Associated with menstruation, these episodic migraine patients experience regular attacks within two days before the menstruation and the first three days of bleeding. The diagnosis of migraine is made almost exclusively from the symptoms described by the patient, since brain changes associated with migraine are subtle and particularly difficult to detect with imaging techniques. Changes in the microenvironment of the tissues can be detected by diffusion-weighted MRI (dMRI). Previous studies using diffusion tensor imaging (DTI) reported lower mean diffusivity (MD) values in migraine patients3,4. However, the signal from the diffusion image voxels contains the contribution, not only from the brain tissue, but also from the free-water (FW) partial volume fraction. The mixture of both contributions may hinder the estimation of the tissue properties. Therefore, some diffusion signal models consider two compartments, one characterized by isotropic free water diffusion and another corresponding to the tissue, using for example DTI. In this work, our objective is to investigate if we gain sensitivity to the alterations in the white matter of menstrual migraine patients from the DTI parameters estimated following elimination of the free water (FW) partial volume fraction estimated using a spherical means (SM) technique.Methods
Diffusion MRI datasets were acquired in a 3T Siemens Vida scanner with a 64 channel receive RF coil from female subjects, 15 healthy (age 31 ± 7 years) and 14 menstrual migraine patients without aura (age 35 ± 8 years). Healthy subjects were studied in one session considering their menstrual cycle: midcycle (after the ovulation). The migraine patients were submitted to a session between migraine attacks (interictal). The diffusion sequence used eight non-diffusion weighted volumes (b = 0 s/mm2) and 3 shells (b = 400, 1000, 2000 s/mm2) along 124 gradient directions (32, 32, 60 respectively for each b value). DESIGNER pipeline5 was used to pre-process the data. FW maps were calculated applying the SM method using dMRI-Lab toolbox; this method was used in order to reduce FW estimation bias in crossing fibre regions6. Subsequently, the diffusion signal was corrected by subtracting the FW partial volume fraction, and DIPY’s TensorModel tool7 was used to calculate the DTI parameters from the corrected diffusion signal: MD and fractional anisotropy (FA). The FW and DTI-derived parametric maps were skeletonized, and the following statistical tests were carried out using voxel-wise FSL’s Tract-based spatial statistics (TBSS)8: i) comparing between the metrics derived from the corrected and original diffusion signal for each subject group using a two-sample paired t-test; ii) comparing tensor parameters (FA and MD) and the FW values of white matter using a two-sample unpaired t-test between the migraine patients and the healthy subject groups. Statistical maps were obtained in each case displaying the regions where significant differences were observed. White matter tracts were identified by using the Johns Hopkins University ICBM-DTI-81 White-Matter Labels Atlas9 provided in the FSL toolbox.Results
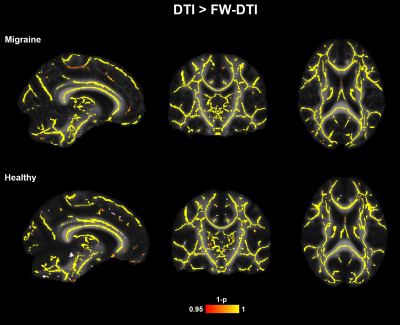
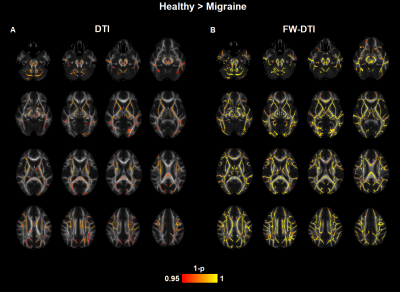
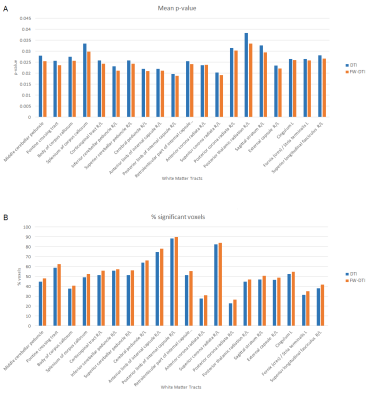
The correction of the diffusion signal by the free-water fraction allows to obtain significantly lower MD values than the MD calculated from the original signal (Figure 1). Comparison between the migraine patients and the healthy subjects presented significant differences in the white matter skeleton for MD, with significantly lower values for the patients in comparison with the controls for both non-corrected diffusion signal (Figure 2A) and corrected (Figure 2B). These differences were found in several regions from the ICBM-DTI-81 White Matter Atlas: 34 regions for MD. When using the MD derived from the corrected diffusion signal to compare between groups, lower p-values were obtained in regions where the differences were significant than when comparing the MD skeletons calculated from the original diffusion-weighted signals (Figure 3).Discussion/Conclusions
The application of the correction to the diffusion signal allows to obtain lower values of MD. Differences between the patients and the healthy subjects were found using MD estimated from standard DTI. However, it was possible to observe a higher number of significant voxels following the correction for FW fraction in the diffusion signal. On the other hand, the lower MD values in menstrual migraine patients suggest abnormal white matter properties. Further work is required to determine which processes could explain these observations.Acknowledgements
This work was supported by Ministerio de Ciencia e Innovación of Spain with research grant PID2021-124407NB-I00 and TED2021-130758B-I00, and Margarita Salas grants for the training of young PhD researchers CA1/RSUE/2021-00801 from Universidad Autónoma de Madrid, Ministerio de Universidades and Plan de Recuperación, Transformación y Resilencia of Spain. We acknowledge the Portuguese Science Foundation through grants PTDC/EMD-EMD/29675/2017, LISBOA-01-0145-FEDER-029675 and UIDB/50009/2020.References
1. Hoffmann et al. Neurovascular mechanisms of migraine and cluster headache. Journal of Cerebral Blood Flow and Metabolism, 2019, 39(4): 573–594.
2. Vetvik et al. Menstrual migraine: a distinct disorder needing greater recognition. The Lancet Neurology, 2021, 20(4):304-315.
3. Yu et al. Axonal loss of white matter in migraine without aura: A tract-based spatial statistics study. Cephalalgia, 2013, 33:34-42.
4. Rocca et al. A diffusion tensor magnetic resonance imaging study ofbrain tissue from patients with migraine. J.Neurol. Neurosurg. Psychiatry 2003, 74:501-503.
5. Ades-Aron et al. Evaluation of the accuracy and precision of the diffusion parameter EStImation with Gibbs and NoisE removal pipeline . NeuroImage 2018; 183:532-543.
6. Tristán-Vega et al. Accurate free-water estimation in white matter from fast diffusion MRI acquisitions using the spherical means technique. Magn Reson Med. 2021; 87:1028–1035.
7. Chang et al. RESTORE: robust estimation of tensors by outlier rejection. Magn Reson Med 2005; 53:1088-95.
8. Smith et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 2006, 31:1487-1505.
9. Oishi et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage, 2008, 43(3):447–457.
Figures