4592
PASTeUR package extension to slab-selective excitations and 3D-EPI for plug-and-play parallel transmit at 7 Tesla1Paris-Saclay University, CEA, CNRS, BAOBAB, NeuroSpin, Gif-sur-Yvette, France, 2Siemens Healthcare SAS, Saint-Denis, France, 3CHU Poitiers, Poitiers, France, 4LRCOM I3M, DACTIM LMA CNRS 7348, University of Poitiers, Poitiers, France, 5Laboratory of Applied Mathematics, UMR CNRS 7348, University of Poitiers, Poitiers, France, 6German Center for Neurodegenerative Diseases (DZNE), Bonn, Germany, 7Department of Physics and Astronomy, University of Bonn, Bonn, Germany
Synopsis
Keywords: Parallel Transmit & Multiband, Parallel Transmit & Multiband, High-Field MRI; fMRI
Keywords: Parallel Transmit & Multiband; High-Field MRI; RF Pulse Design & Fields; fMRI
PASTeUR is a package of sequences using non-selective parallel transmission Universal Pulses (UP) as a plug-and-play pTX solution alleviating B1+ inhomogeneities. Here, we extend the UP solution to slab-selective excitations while preserving a plug-and-play philosophy. A slab of any thickness/orientation/rotation can be acquired without subject-specific calibrations. In vivo experiments performed with an fMRI protocol on a 3D-EPI show that the improved B1+ homogeneity can result in higher SNR. Moreover, the slab-selective UP coupled with parallel imaging and segmented k-space acquisitions pushed further the quality of 3D-EPI.
Introduction
Parallel transmission (pTX) is currently one of the best approaches to alleviate B1+ field inhomogeneities, but usually comes with a cumbersome workflow. To address this challenge, the PASTeUR1 package is a set of non-selective 3D sequences using the pTX Universal Pulses2 (UP) solution including 3D GRE, MPRAGE, MP2RAGE and a SPACE with FLAIR and DIR modules. In this work, the slab-selective UP3 (Slab-UP) were developed. Following the original plug-and-play philosophy of UP, a single slab-UP has been designed to outperform the CP mode slab for any slab thickness, orientation, and position. The performance of slab-selective UP was tested in vivo using state-of-the-art 3D-EPI4,5 and 3D-GRE MR sequences, which will be included in the next PASTeUR release.Methods
To avoid a cumbersome library of RF pulses for each slab thickness/position/orientation, a single universal kT-point pulse6,7 was designed to be employed on the whole brain (database of ΔB0/ B1+ field maps of 20 healthy subjects, 3 kT-points with sub-pulse duration: 920 µs, total duration: 3.78 ms). The RF energy limits were chosen such that the square pulses could be replaced by high-time bandwidth product sinc pulses (up to 25) without exceeding hardware limitations (in particular the peak power). A dedicated sequence module dynamically generates a slab-selective gradient shape and carrier frequency according to the field of view (FOV) thickness, position and orientation chosen by the operator.Acquisitions were performed on three healthy volunteers on a 7 Tesla Magnetom Terra system (Siemens Healthcare, Erlangen, Germany) equipped with an 8Tx/32Rx head coil (Nova Medical, Wilmington, MA, USA). The Slab-UP was first injected into an Actual Flip Angle (AFI) sequence to evaluate its imaging performance. A large slab covering the entire brain was acquired on two volunteers with flip angles of respectively 15° and 30°. The Slab-UP was then inserted into a 3D-EPI sequence and tSNR (temporal SNR) maps were acquired at 1.6 mm3 isotropic resolution (TRvol/TE = 1.2s/26ms, FOV 206 mm, PF = 7/8, GRAPPA 2×4 with blipped-CAIPI8 z-shift 2, 55 repetitions), in slab-selective CP mode and pTX slab-UP mode (48 slices), as well as non-selective CP and pTX (UP pulse from PASTeUR package) modes (100 slices). Different nominal flip angles were used, as specified in the following figures. Segmented, undersampled k-space acquisitions5 at 0.8 mm3 isotropic resolution were also performed with slab-selective CP and pTX Slab-UP modes (TR/TE = 47.2/19 ms, FOV 210 mm, 90 slices, PF = 6/8, GRAPPA 3×1, segmentation factor 3). The acquisition time per slab was 12.7 s. Lastly, high-resolution susceptibility-weighed imaging (SWI9) was performed on a single volunteer with a slab-UP 3D GRE sequence (acq time: 5 min, TR/TE = 28/20 ms, FA = 15°, spatial resolution: 0.3x0.3x3 mm3, GRAPPA 3).
Results
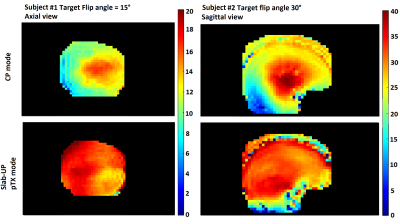
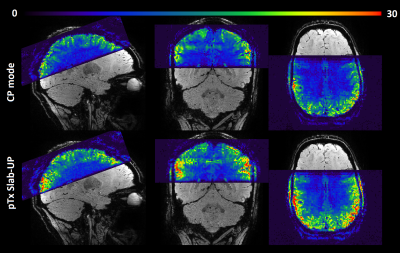
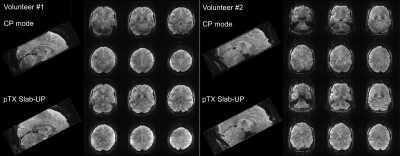
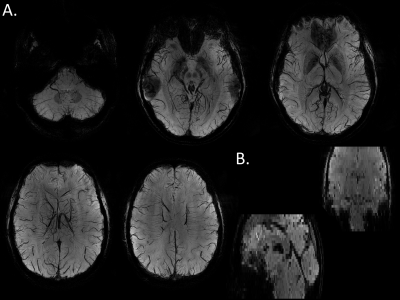
Figure 1 shows AFI flip angle maps for the two volunteers in CP and pTX in slab-selective excitation modes. The Slab-UP mode yields a significantly more homogeneous flip angle distribution throughout the brain compared to the CP mode. The gap in performance is higher in the cortical areas of the brain and particularly in the cerebellum. Figure 2 displays tSNR maps acquired on the first volunteer in CP mode and pTX UP, in non-selective and slab-selective modes, with a nominal flip angle of 10°. The flip angle undershoot yielded by the CP mode in most parts of the brain resulted in a weaker tSNR than in pTX mode for both non-selective and slab-selective excitations. The difference in tSNR is the largest in the cerebellum, where the CP mode flip angle was very small. Figure 3 shows tSNR maps from acquired slabs (flip angle: 5°) on the second volunteer in CP and pTX modes. The slab is overlaid on a high-resolution anatomical image acquired with the 3D-EPI using the segmented k-space5 acquisition and non-selective UP excitation. The slab fits perfectly the FOV with no aliasing suggesting that the slab-selective gradient and carrier frequency are correctly generated. Similarly to Figure 2, the tSNR is higher in pTX thanks to an improved B1+ homogeneity. Figure 4 shows the slab with a high-resolution segmented k-space acquisition. The use of the slab-UP allowed to improve signal intensity in the cerebellum for both volunteers. Finally, Figure 5 illustrates high image quality obtained for SWI (minimum-intensity projection) at various slice positions. Note that image signal and contrasts are maintained even in the cerebellum.Discussion and conclusion
This work reports the development of Universal Pulses extension to slab-selective excitations and their experimental validation in 3D-EPI and 3D-GRE sequences. This new feature allows benefitting from a reduced acquisition time thanks to the slab excitation as well as an improved B1+ field homogeneity thanks to the UP at the same time. For 3D-EPI, the protocols used for the tSNR mapping were close to conventional fMRI protocols; the use of Universal Pulses was very beneficial SNR-wise. For SWI, the slightly increased duration and RF power of pTx Slab-UP did not require modifying sequence parameters. Following these positive results, the next PASTeUR package release will include slab-UP for all already available MR sequences, as well as a 3D-EPI sequence.Acknowledgements
ERPT equipment program of the Leducq Foundation and FET-Open AROMA grant agreement n° 885876.References
[1] Massire et al. (2022). PASTEUR: Package of anatomical sequences using parallel transmission universal pulses now available for MAGNETOM Terra. Siemens Healthineers MAGNETOM Flash (80).
[2] Gras et al. (2017). Universal pulses: A new concept for calibration‐free parallel transmission. MRM 77(2), 635–643.
[3] Jamil et al. (2021). General gradient delay correction method in bipolar multispoke RF pulses using trim blips. MRM 85(2), 1004–1012.
[4] Poser et al. (2010). Three-dimensional echo-planar imaging at 7 Tesla. NeuroImage, 51(1), 261–266.
[5] Stirnberg et al. (2021). Segmented K-space blipped-controlled aliasing in parallel imaging for high spatiotemporal resolution EPI. MRM 85(3), 1540–1551.
[6] Cloos et al. (2012). kT-points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. MRM 67(1), 72–80.
[7] Gras et al. (2015). Joint design of kT-points trajectories and RF pulses under explicit SAR and power constraints in the large flip angle regime. JMR 261, 181–189.
[8] Setsompop et al. (2012). Blipped-Controlled Aliasing in Parallel Imaging for Simultaneous Multislice Echo Planar Imaging with Reduced g-Factor Penalty. MRM 67(5), 1210–1224.
[9] Haacke et al. (2004). Susceptibility weighted imaging (SWI). MRM 52(3), 612–618.
Figures