4590
Reproducibility and variability of tailored and universal nonselective excitation pulses at 7T for human body imaging: Re-scans after one year1Physikalisch-Technische Bundesanstalt (PTB), Berlin and Braunschweig, Germany, 2Working Group on Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center, a joint cooperation between the Charité Medical Faculty and the Max-Delbrück Center for Molecular Medicine, Berlin, Germany, 3Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States
Synopsis
Keywords: RF Pulse Design & Fields, Parallel Transmit & Multiband
3D ultrahigh field (UHF) imaging of the body, particularly the heart, is highly challenging due to the inhomogeneous transmit field (B1+) leading to spatially varying flip-angle (FA) patterns. This study demonstrates that pre-computed universal pulses (UPs) allow for calibration-free 3D cardiac FA homogenization at 7T, despite inter-subject variations because of sex, age, BMI, and coil-placement differences. Furthermore, the performance is robust across multiple MRI operators or re-scans after one year. UPs are, therefore, suited to significantly simplify UHF cardiac imaging by removing the need for lengthy calibration prescans.Introduction
Ultrahigh field (UHF; >3T) MRI of the human body is limited by variations in spatial flip angle (FA) patterns.1 In contrast to subject-tailored parallel transmission (pTx) methods2,3, requiring subject-specific B1+-maps4, we demonstrated that calibration-free cardiac universal pulses (UPs) are applicable in the body, thus saving valuable scan time.5 When generating the B1+-library for cardiac UPs calculation, we found substantial variations in the channel-wise B1+-distributions among subjects due to i) different body sizes and shapes and ii) different placements of the pTx coil on the body. Due to this effect, our UP library for the heart required more subjects than the brain UP counterpart.6 Interestingly, however, UPs calculated for the heart resulted in good FA homogeneity within the heart. UPs also performed well in surrounding tissue, such as the aorta, which was unexpected but consistently observed in all volunteers.5 This indicates a certain robustness of the UPs also for different placements of the RF coil, which is needed for clinical applications. In this work, we analyze the reproducibility and variability of three different excitations in seven subjects: default excitations, subject-tailored pTx and calibration-free UPs. To this end, a different MRI operator re-scanned the seven subjects after one year.Methods
MRI was performed on a Magnetom 7T scanner (Siemens Healthineers, Erlangen, Germany) with a certified commercial 32-element body array (MRI.TOOLS, Berlin, Germany) driven in 8Tx/32Rx mode. 36 channel-wise 3D B1+-datasets were acquired under free-breathing2 and were divided into three groups: i) UP-library (group1: 14M/8F, 21-66years, 19.8-28.3kg/m2), ii) unseen test-cases (group2: 3M/4F, 25-56years, 19.5-35.3kg/m2) and iii) re-scans of unseen test-cases (group3: same group as group2; re-scanned after one year). Subject-tailored 4kT-point pulses (TP) were designed for each dataset of group2, and a 4kT-point UP was calculated using 22 B1+-datasets of group1.5 The TPs/UP were then re-applied to group3 approximately one year later. The posterior half of the coil was centred on the volunteer's heart in the head-to-foot direction, while the anterior half was placed 2cm from the volunteer's chin based on anatomical landmarks and volunteer feedback. A different MRI operator performed the coil placement of group3. The reproducibility and variability of three excitations were assessed among groups2&3: the default excitation (a default shim for the heart set by the vendor), TP and UP. Reconstruction of B1+-datasets, manual slice-by-slice selection of 3D cardiac ROIs, design of RF pulses, and generation of pulse files was performed on a separate workstation. The pulses' performance was analyzed using FA predictions and coefficient-of-variations (CV) in the heart volumes.3 The source code and the B1+-datasets can be downloaded from https://github.com/chaigner/UP_body.Results and Discussion
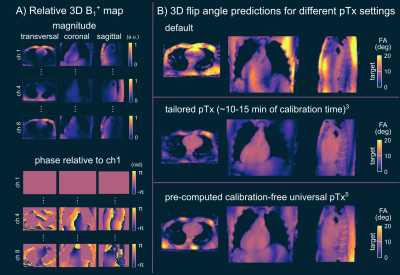
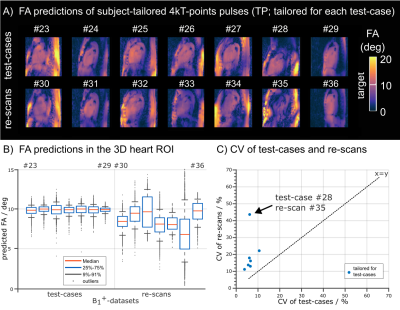
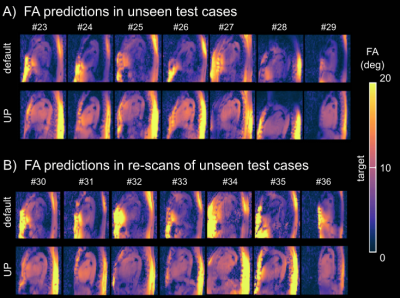
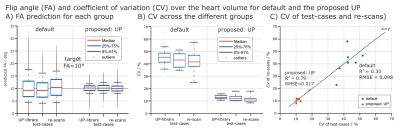
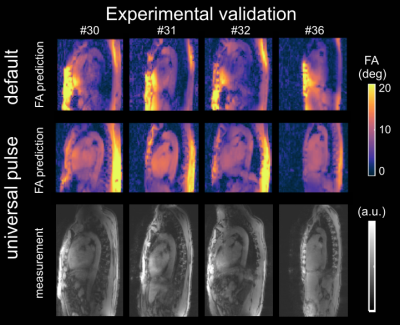
Fig.1 shows relative channel-wise 3D B1+-datasets of the thorax and the FA prediction of three different pTx settings (default, tailored and UP) in the human heart for one representative B1+-datasets of group1 (UP-library). Fig.2 shows the predicted FAs and the CV in the heart volume using subject-tailored 4kT-points pulses (TP). Note the substantial differences in spatial FA variations resulting from a variation of the heart size, heart position, BMI, and coil placement between the datasets. Applying the same TP to group3 resulted in elevated FA spread (Fig.2B) and increased CV values (Fig.2C) in all re-scans. The median CV of 6.3% (test-cases) increased to 19.73% (re-scans) demonstrating the need to re-acquire B1+-maps and re-calculate the pTx-pulses in the TP case. Moreover, the TP resulted in substantial FA dropouts in the heart in re-scan #35, likely caused by different coil placement. Fig.3 shows the FA prediction using the default RF shim and the UP for a sagittal slice of the 3D volume. In all test-cases and re-scans, the default RF shim resulted in FA dropouts in the FA predictions that were prevented by applying the UP. This was also the case for re-scan (#35), with substantial differences in the coil placements. Fig.4A shows the quantitative FA predictions in the heart volume using two pTx settings (default and UP). The default RF shim and the UP achieve a median FA close to the target FA of 10° but result in a diverse FA spread. As expected, the UP outperforms the default RF shim with higher minimal FAs and a reduced FA spread from 6.2° to 1.8° (interquartile range). Fig.4B-C show the CV evaluation in the heart volume. Across all 36 datasets, the UP reduces the CV below 20% with a median CV of 12.4% (range=8.2%-18.6%) compared to the default phase setting with a median CV of 42.8% (range=23%-64.8%). Across the different B1+-dataset groups, the UP consistently delivers CV values of approximately 12% (UP-library: 12.7%, test-cases: 11.8% and re-scans: 11.5%). Figure 5 shows exemplary 3D GRE images (without cardiac gating) from four re-scans using the proposed UP. Close agreement was observed between the B1+-predictions and the 3D GRE images, demonstrating coil-placement robustness of calibration-free pTx in the human heart.Conclusion
Despite substantial B1+-variations between scan and re-scan, the work shows that pre-computed UPs provide robustness not only against FA variations among subjects but also under changes in coil placements. This highly valuable property was not the case for the static default shim or the TPs and makes the UP suitable for calibration-free 3D body applications at 7T.Acknowledgements
We gratefully acknowledge funding from the German Research Foundation SCHM 2677/2-1, SCHM 2677/4-1 and GRK2260, BIOQIC.References
[1] Ladd, E, Bachert, P, Meyerspeer, M, et al. Pros and cons of ultra-high-field MRI/MRS for human application, Prog. Nuc. Magn. Reson. Spec. 2018; 109:1-50. doi: 10.1016/j.pnmrs.2018.06.001
[2] Padormo, F, Beqiri, A, Hajnal, JV, and Malik, SJ. Parallel transmission for ultrahigh‐field imaging. NMR Biomed. 2016; 29: 1145– 1161. doi: 10.1002/nbm.3313
[3] Aigner, CS, Dietrich, S, Schmitter, S. Three-dimensional static and dynamic parallel transmission of the human heart at 7 T. NMR in Biomedicine. 2021; 34:e4450. doi: 10.1002/nbm.4450
[4] Dietrich, S, Aigner, CS, Kolbitsch, C, et al. 3D Free-breathing multichannel absolute B1+ Mapping in the human body at 7T. Magn Reson Med. 2021; 85: 2552– 2567. doi: 10.1002/mrm.28602
[5] Aigner, CS, Dietrich, S, Schaeffter, T, Schmitter, S. Calibration-free pTx of the human heart at 7T via 3D universal pulses. Magn Reson Med. 2021; 87: 70– 84. doi: 10.1002/mrm.28952
[6] Gras, V, Vignaud, A, Amadon, A, Le Bihan, D and Boulant, N Universal pulses: A new concept for calibration‐free parallel transmission. Magn. Reson. Med. 2017; 77: 635-643. doi: 10.1002/mrm.26148
Figures