4575
Diffusion Weighted Imaging using a Prostate Nonlinear Gradient Coil1Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Magnetic Resonance, Siemens Healthcare GmbH, Erlangen, Germany, 3Università Cattolica del Sacro Cuore, Rome, Italy, 4Biomedical Engineering, Yale University, New Haven, CT, United States
Synopsis
Keywords: Gradients, Prostate
This work presents the early performance characteristics of an inside-out prostate nonlinear gradient (NLG). Diffusion-weighted imaging (DWI) could detect prostate cancers non-invasively. However, it requires high b-values, which in turn require a gradient pulse with a long duration, increasing the echo time. A longer echo time means a lower SNR and lower contribution from short T2 components. NLG coil circumvents this issue by having a gradient with a high amplitude within a limited field of view, which applies to prostate imaging.Introduction
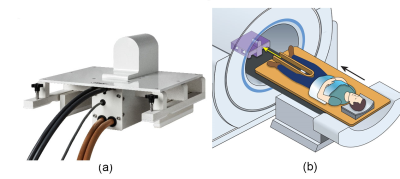
The lifetime risk of being diagnosed with prostate cancer is approximately 1/9 for American men, but because of slow progression and the significant morbidities associated with therapy, treatment for many cases can often be declined or delayed.1 Moreover, prostate cancer is considered the second leading cancer killer of men, so the early detection of aggressive prostate cancers is crucial. Accordingly, a sensitive imaging method that distinguishes benign or low-grade prostate lesions from aggressive ones is one of the greatest needs in prostate cancer. Diffusion-weighted imaging (DWI) is currently one of the best ways to detect prostate cancers non-invasively.2 According to the new PI-RADS guidelines 3, it is recommended to acquire a few DWIs, one of which has a b-value ≥ 1400 s/mm2. While using linear gradients, such a high b-value would require a gradient pulse with a long duration which will increase the echo time and, in turn, that will compromise the image SNR. However, using a nonlinear gradient (NLG) coil can circumvent this issue by having a gradient with a high amplitude within a limited field of view, which applies to prostate imaging. Some earlier work investigated the feasibility of using non-linear gradients with diffusion in 4. Here we present some initial performance characteristics of an inside-out nonlinear gradient (shown in Figure 1) for prostate imaging and the feasibility of DWI and ADC mapping using a polyvinylpyrrolidone (PVP) phantom.Methods
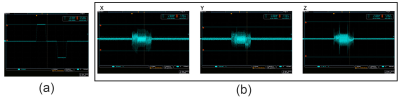
Initial performance characteristicsHeating and vibrations, as well as inductive coupling, were assessed. In addition, B1 mapping was performed using a dual flip angle sequence with and without the presence of the gradient.
Phantom DWI experiments
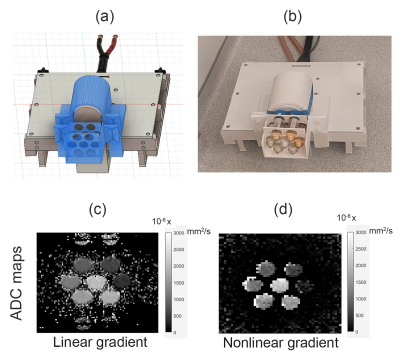
We created a PVP water solution phantom with different PVP concentrations, which is considered a good candidate for a non-toxic diffusion MR phantom.5 Different concentrations of PVP were created by adding PVP40 (Sigma-Aldrich, average wt 40,000) to distilled water, ranging from 5% to 50% w/v. Diffusion data of the phantom was acquired twice, once with the NLG coil and another time with the linear gradient coil for validation. The linear gradient scan consisted of b-values of 0, 200, 400, and 600 s/mm2. The b-values of the NLG vary based on the location, and three DWI were used for ADC fitting.
Results
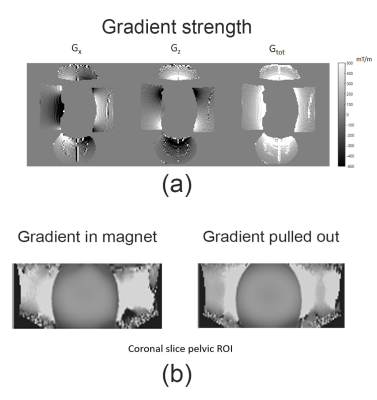
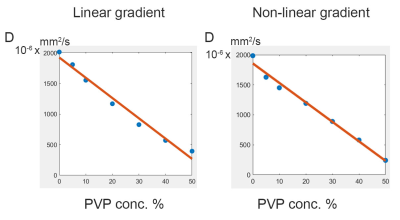
Neither heating nor vibration was observed in initial studies. Inductive coupling from linear gradients was also tested and appeared to generate <10V peak voltage (see Figure 2b). Figure 3a shows the detected gradient strength scaled to 320A of current. Other studies (not shown) have verified linearity with amplitude with current input. As expected, gradients of 500mT/m are detected in the HF direction inferior to the coil, where prostate ROI is targeted. Preliminary B1 maps (Figure 3b) indicate that the gradient does not significantly affect excitation. Figures 4.c and 4.d show the ADC maps calculated using scans with linear and nonlinear gradient coils respectively. In addition, Figure 5 shows that both scans produced the expected inverse linear relationship between the diffusivity and the PVP concentrations.Discussion and conclusion
Initial experience with a new inside-out gradient design for high-gradient strength in prostate DWI is promising. No heating or vibration was observed. Field shape and gradient strength are well aligned with design specifications delivering strong gradients in the region of interest. In addition, the non-linear gradient coil produced similar diffusivity results with different PVP concentrations to those produced using the linear gradient coil. The non-linear gradient coil produced similar diffusivity results with different PVP concentrations to those produced using the linear gradient coil.Acknowledgements
No acknowledgement found.References
1. Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA: A Cancer Journal for Clinicians 70, 7-30, doi:https://doi.org/10.3322/caac.21590 (2020).
2. Rud, E. & Baco, E. Re: Jeffrey C. Weinreb, Jelle O. Barentsz, Peter L. Choyke, et al. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol 2016;69:16-40: Is Contrast-enhanced Magnetic Resonance Imaging Really Necessary When Searching for Prostate Cancer? Eur Urol 70, e136, doi:10.1016/j.eururo.2016.04.017 (2016).
3. Kwon, M. R., Kim, C. K. & Kim, J. H. PI-RADS version 2: evaluation of diffusion-weighted imaging interpretation between b = 1000 and b = 1500 s mm(-)(2). Br J Radiol 90, 20170438, doi:10.1259/bjr.20170438 (2017).
4. Feldman, R. E. et al. Results for diffusion-weighted imaging with a fourth-channel gradient insert. Magn Reson Med 66, 1798-1808, doi:10.1002/mrm.22971 (2011).
5. Pierpaoli, C., Sarlls, J., Nevo, U., Basser, P. J. & Horkay, F. in ISMRM 2009.
Figures