4561
Open-Bore Hybrid MPI-MRI Scanner: A Proof-of-Concept Simulation Study1Aselsan Research Center, ASELSAN A.Ş., Ankara, Turkey, 2Electrical and Electronics Engineering, Bilkent University, Ankara, Turkey, 3National Magnetic Resonance Research Center (UMRAM), Bilkent University, Ankara, Turkey
Synopsis
Keywords: Hybrid & Novel Systems Technology, Low-Field MRI, Magnetic Particle Imaging
Magnetic particle imaging (MPI) is a novel technique that images background-free spatial distribution of magnetic nanoparticles inside the body. The lack of signal from the background tissue provides high sensitivity to MPI. However, anatomical information is also required in many applications. Here, we present an open-bore hybrid preclinical MPI-MRI system, in which the coils can be utilized interchangeably between MPI and MRI data acquisitions. We analyze the imaging performance for both modalities using a simulation model based on our in-house prototype MPI system that can generate 0.5 T/m selection field gradients for MPI and 50 mT B0 for low-field MRI.INTRODUCTION
Magnetic particle imaging (MPI) is an emerging tracer-based imaging technique, which provides the concentration distribution of magnetic nanoparticles (MNPs) without using ionizing radiation1. MPI exploits the non-linear magnetization response of MNP tracers injected into the body. Since biological tissues do not possess such a magnetic behavior, MNPs are the only source of signal in MPI2. Although the lack of background signal provides high sensitivity and excellent contrast capabilities to MPI, anatomical information is missing in the resultant images. Therefore, an additional imaging modality, such as MRI or CT, is needed to obtain an anatomical image. Fusion of MPI and MRI images have been achieved previously, using separate3 or hybrid system configurations4-7. Utilizing separate MRI and MPI systems is suboptimal for clinical applications due to patient transportation requirement between two imaging sites, as well as possible artifacts in image fusion. Hybrid MPI-MRI systems have been proposed to overcome such problems. Current hybrid systems are in closed-bore configuration. While these systems provide high magnetic field efficiency, they do not allow interventional operations and may cause discomfort to patients. We recently proposed an open-sided MPI scanner8, which is capable of electronical rotation and translation of a field-free-line (FFL). The performance of the imaging system was validated via both simulation9,11 and experimental studies10. Here, we present an open-bore hybrid preclinical MPI-MRI system, where the coils can be utilized interchangeably between MPI and MRI modes. With a proof-of-concept simulation study on our prototype MPI hardware, we demonstrate the imaging performance of the hybrid system with 0.5 T/m selection field gradients for MPI and 50 mT B0 field for low-field MRI.METHODS
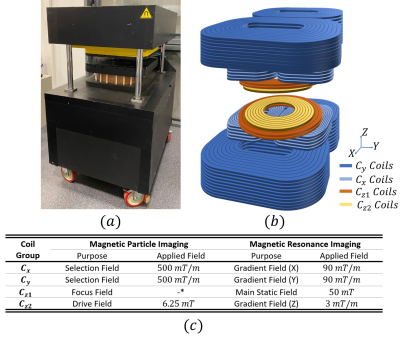
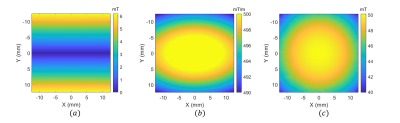
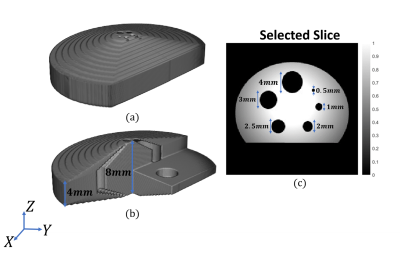
MPI uses three types of magnetic fields for imaging: selection-field (static gradient field with a field free region that enables spatial resolution), focus-field (homogeneous slowly-varying magnetic field to move the field free region in the imaging volume), and drive-field (homogeneous excitation field at 10-30 kHz to induce time-varying MNP magnetization). In our prototype system shown in Fig. 1(a)-(b), selection field of MPI is generated using two bi-planar gradient coil groups, denoted as Cx and Cy, which generate an FFL in x-y plane8. FFL can be rotated on the x-y plane by adjusting the coil currents8,9. These Cx and Cy coil groups can be used to generate the x- and y-gradient fields of MRI, respectively. A Helmholtz configuration coil group, denoted as Cz1, generates the focus-field for MPI, which can be used to generate the main static field (B0) for MRI. Another coil group, denoted as Cz2, generates the drive-field of MPI when used in Helmholtz configuration, and can be used to generate the z-gradient-field of MRI when used in Maxwell configuration. Employment of each coil group for both MPI and MRI configurations, as well as the performance for each configuration, are given in Fig. 1(c).For the simulations in this work, the magnetic field maps of the Cx, Cy, and Cz1 coils (Fig. 2) were obtained via Maxwell (Ansys Electromagnetics Suite, Ansys Inc., USA) simulations. These field maps were then imported into in-house MRI and MPI simulators developed in MATLAB. The receive coil of MPI and the Tx/Rx coils of MRI were assumed to be ideal. For MPI/MRI simulations, a field-of-view (FOV) of $$$25\times25\times15 mm^3$$$ was discretized with $$$0.2 mm$$$ isotropic grids. For each grid, a magnetic moment vector was assigned, and Bloch equations were applied to simulate free-induction decay and rotations due to RF pulses, off-resonance effects, and gradient fields. For the MRI simulation, a spin-echo sequence was implemented with line-by-line k-space acquisition. The imaging parameters were 0.3 mm isotropic in-plane resolution and 3 mm slice thickness. The imaging performance of the MRI configuration was analyzed for different inhomogeneity levels of the B0 field. For the MPI simulations, 0.5 T/m selection field gradient and 6.25 mT-peak, 25 kHz drive-field were applied. For the MPI image reconstruction, system matrix-based reconstruction method was used with the Kaczmarz algorithm11. The reconstruction results for a 3D in-silico phantom (Fig. 3) were obtained for different scenarios to observe the system-induced deviations.
RESULTS & DISCUSSION
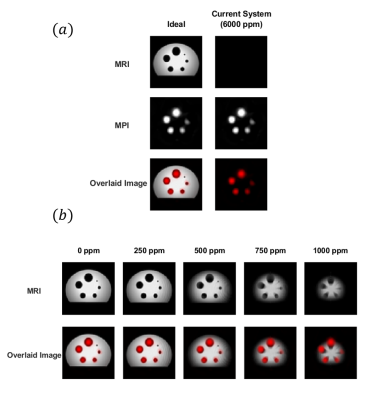
Figure 4(a) shows the reconstruction results when ideal magnetic fields vs. the magnetic fields of the current prototype system are utilized. The results show that the current prototype system provides near-ideal performance for MPI, but does not have sufficient performance for MRI as is. The off-resonance effects due to B0 field from Cz1 are at ~6000 ppm level, causing rapid dephasing of the MRI signal. To determine the level of homogeneity needed for the MRI configuration, the off-resonance map of the Cz1 coil was scaled down and the MRI simulations were repeated. As shown in Fig. 4(b), the results indicate that inhomogeneities beyond 750 ppm result in significant dephasing and slice positioning issues, whereas inhomogeneity levels between 250-500 ppm lead to successful MRI acquisitions. This relatively moderate homogeneity requirement can be addressed by incorporating an additional shim coil to our current prototype system.CONCLUSION
We have presented an open-sided hybrid preclinical MRI-MPI system with dual-mode coil configuration. The proof-of-concept simulation results show that the current system has near-ideal MPI performance, and can achieve successful low-field MRI acquisitions by adding a shim coil for B0 field homogenization.Acknowledgements
No acknowledgement found.References
1. Gleich, B., & Weizenecker, J. (2005). Tomographic imaging using the nonlinear response of magnetic particles. Nature, 435(7046), 1214–1217. doi: 10.1038/nature03808
2. Zheng B, Yu E, Orendorff R, Lu K, Konkle JJ, Tay ZW, Hensley D, Zhou XY, Chandrasekharan P, Saritas EU, Goodwill PW, Hazle JD, Conolly SM. Seeing SPIOs Directly In Vivo with Magnetic Particle Imaging. Mol Imaging Biol. 2017 Jun;19(3):385-390. doi: 10.1007/s11307-017-1081-y. PMID: 28396973; PMCID: PMC5724571.
3. J. Weizenecker, "Three-dimensional real-time in vivo magnetic particle imaging", Phys. Med. Biol., vol. 54, pp. L1-L10, 2009.
4. J. Franke et al., "First hybrid MPI-MRI imaging system as integrated design for mice and rats: Description of the instrumentation setup", Proc. Int. Workshop Magn. Part. Imag. (IWMPI), pp. 1, Mar. 2013.
5. P. Vogel et al., "MRI meets MPI: A bimodal MPI-MRI tomograph", IEEE Trans. Med. Imag., vol. 33, no. 10, pp. 1954-1959, Oct. 2014.
6. P. Klauer, P. Vogel, M. A. Ruckert, W. H. Kullmann, P. M. Jakob and V. C. Behr, "Bimodal TWMPI-MRI hybrid scanner—Coil setup and electronics", IEEE Trans. Magn., vol. 51, no. 2, pp. 1-4, Feb. 2015.
7. J. Franke et al., "System characterization of a highly integrated preclinical hybrid MPI-MRI scanner", IEEE Trans. Med. Imag., vol. 35, no. 9, pp. 1993-2004, Sep. 2016.
8. Top CB, Ilbey S, Guven HE, Electronically rotated and translated field-free line generation for open bore magnetic particle imaging, Med Phys, 44 (2017) 6225–6238.
9. Top, C.B., Güngör, A., Ilbey, S., & Güven, H.E. (2019). Trajectory analysis for field free line magnetic particle imaging. Medical Physics, 46, 1592–1607.
10. Top CB, Gungor A. Tomographic Field Free Line Magnetic Particle Imaging With an Open-Sided Scanner Configuration. IEEE Trans Med Imaging. 2020 Dec;39(12):4164-4173. doi: 10.1109/TMI.2020.3014197. Epub 2020 Nov 30. PMID: 32746156.
11. D. A. Soydan, A. Güngör and C. B. Top, "A Simulation Study for Three Dimensional Tomographic Field Free Line Magnetic Particle Imaging," 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), 2021, pp. 3701-3704, doi: 10.1109/EMBC46164.2021.9631111.
Figures