4550
Benchtop NMR for lab-on-a-chip
Marc Azagra1, Alejandro Portela1, Hetal Patel2, Dian Weerakonda2, Jose Yeste1, Alba Herrero-Gómez1, Matthew Fallon2, Megdouda Benamara3, Marc Dubois3, Tryfon Antonakakis3, Javier Ramon1, and Irene Marco-Rius1
1Institute for Bioengineering of Catalonia, Barcelona, Spain, 2Oxford Instruments, Abingdon, United Kingdom, 3Multiwave Imaging, Marseille, France
1Institute for Bioengineering of Catalonia, Barcelona, Spain, 2Oxford Instruments, Abingdon, United Kingdom, 3Multiwave Imaging, Marseille, France
Synopsis
Keywords: New Devices, Hyperpolarized MR (Non-Gas), Molecular Imaging
A benchtop NMR spectrometer has been designed and optimized to monitor real-time metabolism of 3D tissue engineered on microfluidic platforms using hyperpolarization by dynamic nuclear polarisation. We show the design process, which included the modification of a commercial benchtop NMR spectrometer, the design and fabrication of a microfluidic platform for a reliable injection of a hyperpolarized substrate and a constant delivery and renewal of the cell media and its integration with a RF coil for transmit/receive, and the design and fabrication of a sample carrier. We also present our preliminary NMR data using this system.
Introduction
Recent advances in tissue engineering offer new approaches to metabolic disease modelling and drug discovery by providing biologically relevant models of tissues and organs in vitro integrated with sensing technology. This technology has the potential to revolutionise the pharmaceutical industry by enabling reliable and high predictive in vitro testing of chemical compounds. The capability to miniaturise microfluidic systems and advanced tissue fabrication procedures have enabled researchers to create multiple 3D in vitro models with a high degree of control over experimental variables for high-content screening applications. In this intersectoral and international collaborative project, we have developed a benchtop NMR spectrometer to accommodate microfluidic platforms containing 3D in vitro models and enable the study of their cellular metabolism in real-time, in situ and non-destructively by hyperpolarization-enhanced NMR (Fig.1). The integration of this technology with tissue engineering systems will be applied to monitor disease and evaluate responses to different stimulus. As a proof-of-concept, we will present the results of the project including a biomimetic model composed of liver spheroids and pancreatic islets.Methods
A 1.4 T commercial benchtop NMR spectrometer (Pulsar, Oxford Instruments) has been modified to allow for planar microfluidic chips to be inserted into the scanner. For this purpose, a carrier was 3D printed from a photopolymer resin to accommodate the chip outside the scanner prior to the NMR experiment, improving usability (Fig.2). A microfluidic chip was designed and fabricated specially for this NMR spectrometer with the following requirements: i) science chamber in the homogenous B0 region of the spectrometer, ii) reliable injection of a hyperpolarized substrate, iii) a constant delivery and renewal of the cell media, iv) and integrated with a RF coil for transmit/receive. The microfluidic chip was composed by a glass substrate and a single layer of polydimethylsiloxane (PDMS) and fabricated following a standard replica molding protocol1. The science chamber was designed to be sealed on the top by a removable round cover glass to enable accessing the cell chamber and then sealing the fluidic system once the cells or engineered tissue have been inserted.Due to the particularities of the geometry of the NMR spectrometer and the direction of its B0 with respect to the chip plane, a Tx/Rx saddle RF coil was designed to maximise SNR and B1 homogeneity in the science chamber. The design was optimized numerically with CST microwave studio (Finite Element Solver). Several parameters such as the number of turns, the height and the diameter of the coils were optimized to reach the highest average B1 amplitude in the science chamber. The copper traces are 35µm thick and placed on 200-um-thick polyimide substrate to represent accurately to the final prototype that would be produced on flexible substrate. For each case the coil was tuned and matched to 15 MHz and B1 field normalized to 1W of input power.
A 3D cell model containing liver spheroids was developed by seeding an immortalised mouse cell line of hepatocytes (AML12) into a carboxymethyl cellulose scaffold2,3. Magnetic susceptibility of both PDMS and carboxymethyl cellulose were measured by waveguide coaxial cell (EpsiMu, Multiwave Technologies, France).
To test the system, hyperpolarized [1-13C]pyruvate will be prepared by dissolution dynamic nuclear polarization as in ref.4 and injected into the microfluidic system containing the cell-laden scaffold to follow its metabolic conversion in real time.
Results and Discussion
A microfluidic chip was fabricated in PDMS with channels (200 µm x 200 µm) interconnecting a cylindrical science chamber (5 mm height x 5 mm diameter) for NMR detection and a passive membrane pump for delivery of hyperpolarized solutions (Fig.3). The pump consists of a cavity of 20 mm in diameter covered by a thin membrane (800 µm) of PDMS functioning as a pressure damper intended to reduce the critical pressure spike produced during the manual injection of the hyperpolarized solution. Neither the material used for the chip nor the scaffold caused susceptibility artifacts. The optimized saddle coil is presented in Figure 4a. It is a 6-turn cylindrical saddle coil with 5.4-mm-height and 5.5-mm-diameter. A scaffold was placed inside the saddle coil (relative permittivity 80 and conductivity 1.2 S.m-1) to load the probe. B1 amplitude field maps (Fig.4B and 4C) represent the efficiency of the coil at the scaffold location. The averaged amplitude in the scaffold volume is 1.15 mT at 15 MHz for 1W of input power. This efficiency approaches 75% of the standard solenoid probe used in regular Pulsar setup. The homogeneity is evaluated in the scaffold volume at 20% of relative standard deviation. Shimming procedures were tested including a 3D spatial field mapping protocol analogous to gradshim. This protocol could deliver a linewidth of less than 0.1ppm (Fig.3C).Conclusions
The equipment developed in this project will be an easy-to-use and low-cost microfluidic platform for NMR studies of biomedical tissue engineering, environmental control and chemical industry.Acknowledgements
This work has received funding from “la Caixa” Banking Foundation (LCF/BQ/ PI18/11630020), MCIN/AEI/10.13039/501100011033 (Ref. PID2020-117859RA-I00 and RYC2020-029099-I) and by “ESF Investing in your future”, the FI Fellowship Programme from AGAUR (Ref. 2021 FI_B_01039), the European Union’s Horizon 2020 research and innovation program (GA-863037), and the BIST – “la Caixa” initiative in Chemical Biology (CHEMBIO).References
[1] Jo BH et al. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000; 9(1), 76
[2] Velasco-Mallorquí F, Rodríguez-Comas J, Ramón-Azcón J. Cellulose-based scaffolds enhance pseudoislets formation and functionality. Biofabrication. 2021;13, 035044
[3] Herrero-Gómez A, Azagra M, Marco-Rius I. A cryopreservation method for bioengineered 3D cell culture models. Biomed Mater. 2022;17(4).
[4] von Morze C et al. Direct assessment of renal mitochondrial redox state using hyperpolarized 13C-acetoacetate. Magn Reson Med. 2018;79:1862.
Figures
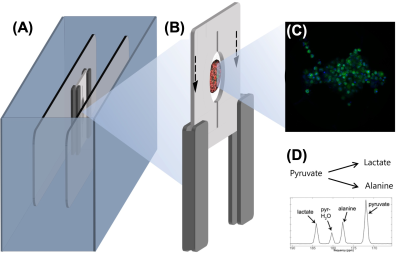
Figure 1. Scheme of the project presented, including a benchtop NMR spectrometer for the detection of hyperpolarisation-enhanced NMR metabolism in 3D tissue engineered cell models: (A) NMR spectrometer, (B) microfluidics chip with embedded RF coil, (C) 3D pancreatic cells, (D) example of metabolic pathway observed upon injected hyperpolarised [1-13C]pyruvate.
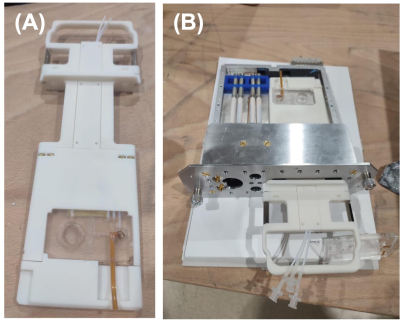
Figure 2. Carrier to ease microfluidic chip insertion into the benchtop NMR scanner probe. (A) 3D printed carrier with microfluidic chip. (B) Demonstration of the carrier fit into the spectrometer’s probe.
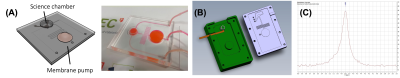
Figure 3. Microfluidic chip designed for a fast and controlled delivery of a hyperpolarized solution while retaining the media renewal capabilities of microfluidic chip bioreactors. (A) Design and fabricated chip. (B) Mould designed to facilitate the fabrication of the chip with an embedded RF coil. (C) NMR signal of water doped with CuSO4 in a chip. The system resolution can be deduced as being better than 0.1ppm as the linewidth is dominated by the intrinsic FWHM of the doped water (~10Hz, 0.17ppm).
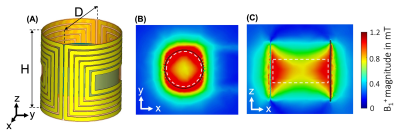
Figure 4. (A) Schematics of the optimized 6-turn cylindrical saddle coil (D = 5.5 mm, H = 5.4 mm). (B) Transverse view of the B1+ field magnitude at 15 MHz. (C) Sagittal view of the B1+ field magnitude at 15 MHz. Simulation results are obtained with 1W of input power. The white dotted line denotes the scaffold region. The saddle coil parameters are optimized to reach an average B1+ amplitude of 1.15 mT with a homogeneity evaluated to 20% (relative standard deviation) within the scaffold region.
DOI: https://doi.org/10.58530/2023/4550