4542
A multiscale approach based on the combination of DTI, XRD and histology to study the myeloarchitecture in a rat model of mTBI1Nanotec, CNR, Roma, Italy, 2IRCSS Santa Lucia Foundation, Roma, Italy, 3University of Eastern Finland,, Kuopio, Finland, 4European Synchrotron Radiation Facility, Grenoble, France, 5Aix-Marseille Université, CNRS, Centrale Marseille, Institut Fresnel, Marseille, France, 6Museo Storico della Fisica e Centro Studi e Ricerche Enrico Fermi, Rome, Italy, 7Russell H. Morgan Department of Radiology and Radiological Science, , John Hopkins University School of Medicine, Baltimore, MD, United States, 8Institute of Crystallography, CNR, Rome, Italy
Synopsis
Keywords: Multimodal, Tissue Characterization, x ray scanning diffraction
We have combined high-resolution structural imaging techniques, such as scanning micro-X-ray diffraction (SμXRD), DTI and histology to study the myeloarchitecture in both healthy and pathological rat brains. For the latter, we focused on an animal model of mild traumatic brain injury (mTBI) that is the most common form of acquired brain injury caused by an external force into the brain. We have performed a quantitative morphometrical analysis of myelin, mapping its relevant morphological and topological parameters (period, orientation, and integrated intensity fluctuation) to assess tissue microstructural damage 35 days after mTBI and sham-operationINTRODUCTION
The ultrastructure of the myelin sheaths is crucial for the function of axons in the brain. Myelinated axons are the main cellular components of white matter, connecting gray matter areas. The myelin ultrastructure has been studied using a wide variety of approaches, such as transmission electron microscopy [1], coherent anti-Stokes Raman scattering microscopy [2] or neutron scattering [3,4]. X-ray diffraction (XRD) is widely used for structural studies of biological tissues [5] and is well suited to study myelin because of the periodic structure of myelinated axons [6,7]. One example is the work of Avila and collaborators, who employed XRD to observe the structural modifications in the myelin sheaths caused by the mutation of the P0 glycoprotein [8]. However, myelin is morphologically heterogeneous, presenting intrinsic local fluctuations in its structural parameters from point-to-point in real space; which makes it quite difficult to probe its intrinsic structural fluctuations with conventional experimental approaches. Indeed, conventional XRD is limited since it only provides insights into the average periodic molecular structure of myelin, probing the k-space (or reciprocal space) with no spatial resolution. Recently, a novel approach based on fast scanning micro-XRD (SμXRD) paired with spatial statistical analysis, was applied to quantify the early changes in the ultrastructure of myelin during the degradation of Xenopus laevis sciatic nerve [9]. In this framework, we applied a multimodal approach with different techniques based on the combination of DTI, SμXRD and histology to examine tissue damage at multiple scales, and provide new insights in the detection of a mild brain injury. We observed that DTI detected minor tissue microstructural changes related to loss of myelinated axons and gliosis, and its limits to detect damage in the ultrastructure of the myelin [10]. Furthermore, the high resolution and the potentiality of the SμXRD, we mapped the morphological and topological parameters characterizing myelin in white matter axons fibers, with a spatial resolution of 1 μm.METHODS
5 Adult male Sprague-Dawley rats were used for all the experiments (10 weeks old, 300-350 g, Harlan Netherlands B.V). All animal procedures were approved by the Animal Ethics Committee of the Province Government of Southern Finland and carried out according to the guidelines set by the European Community Council Directives 2010/63/EEC. The experimental procedures are described in [11].a) Ex-vivo DTI acquisition: We scanned the fixed rat brains on an 11.7 T NMR spectrometer (Bruker BioSpin, MA, USA) with a Micro2.5 gradient system (maximum gradient strength = 1000 mT/m). We used a birdcage volume coil (diameter = 20 mm), a 3D weighted gradient- and spin-echo (DW-GRASE) sequence (TE = 33 ms, TR =800 ms, rare-factor/EPI factor = 4/3, number of averages = 2, bandwidth = 100 kHz, matrix size = 152 × 112 × 78, FOV = 22.8 × 16.8 × 11.7 mm3), b0 images = 4, 30 directions (Δ = 12 ms, δ = 5 ms, b-value = 3000 & 6000 s/mm2 ), resolution of 0.15 mm3 isotropic (zero-filling interpolation to 0.075 mm3 isotropic) scan time of ∼21 h.
b) Histology: We first stained 30-µm-thick coronal brain sections with Nissl (thionin) staining to assess the cytoarchitectonics, and second, with gold chloride staining to assess axonal and myelin damage.
c) SμXRD: We selected one section per animal at -2.00 mm from bregma, rostrally to the lesion site (-3.60 mm from bregma). SμXRD measurements were performed on the ID13 beamline of the ESRF (Grenoble, France). The X-ray beam was monochromatized, and then focused on 2 × 2 μm2 spot with energy of 12.6 keV. The samples were scanned by a step motor stage with 0.5 µm repeatability, using a step size of 2 μm in both the vertical, z, and the horizontal, y, direction with an exposure time of 0.01 s to minimize radiation damage. In each point, we collected a 2D diffraction frame in transmission geometry using a fast Eiger 4 M detector (Dectris) (Figure 1).
RESULTS & DISCUSSION
Myelin is a dual factor and crucial actor in post-TBI axonal changes. We introduced SμXRD to study non-invasively the myelin structure. Indeed, myelin periodic multilamellar structure it is a quasi-crystalline element. SμXRD allows us to get some insights in how the myelin evolves post-TBI. We have selected the same ROIs for the DTI, histology and diffraction analyses in the external capsule, corpus callosum and internal capsule, as reported in Figure 2. We observed that in all the mTBI animals, fractional anisotropy (FA) values appeared lower as compared to sham-operated ones in the external capsule, which is the closest areas to the primary lesion (Figure 3). The XRD profiles obtained in the mTBI animals might indicate different responses to the brain injury, as well as different ongoing degenerative and repair processes in this experimental time point, as suggested by the orientation degree "rho" of the myelin lamellar phase reported in Figure 4CONCLUSIONS
We have shown that at different scale it is possible to have a deep analysis of the effect of the lesion. The obtained results were essential to the understanding of white matter alterations at different stages, which, in turn, might be crucial for the early diagnosis of axonal degeneration in clinics.Acknowledgements
This study was carried out with the support of Kuopio Biomedical Imaging Unit, University of Eastern Finland, Kuopio, Finland (part of Finnish Biomedical Imaging Node, EuroBioImaging). We acknowledge Maarit Pulkkinen for her technical assistance in animal handling and histology, and Dr. Ali Abdollahzadeh for his assistance with the automated cell counting code. We are also grateful for their access to the ID13 beamline at the European Synchrotron Radiation Facility (ESRF) at Grenoble (France).
References
[1] J.B.Finean, (1960), J.Biophysic.And Biochem.Cytol.8, 13–29
[2] H.Wang et al, (2005), Biophys.J.89, 581–591
[3] W.Knoll, et al. (2014), Soft Matter 10, 519
[4] F.Natali, et al. (2013), AIP Conf. Proc. 1518, 551–557
[5] G. Ciasca, et al. (2011), J. Nanopart. Res. 13, 6149–6155
[6] X. Y. Luo, et al. (2008), J. Struct. Bio. 162, 170–183
[7] Aggarwal, D. et al. (2009), J. Struct. Bio. 168, 521–526
[8] R.L. Avila, et al. (2005). J. Neuropathol. Exp. Neurol. 64, 976–990
[9] Campi, G., et al. (2017). ACS nano, 12(1), 729-739.
[10] San Martin Molina et al. (2022) ). J. Neuropathol. Exp. Neurol.,nlac100, https://doi.org/10.1093/jnen/nlac100
[11] San Martín Molina I, et al. (2020) eNeuro 7(3), doi:10.1523/ENEURO.0476-19.2020.
Figures
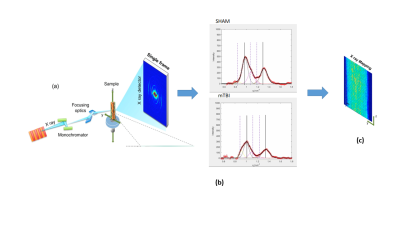
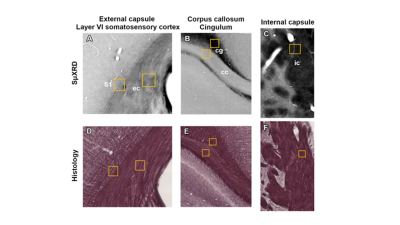
Figure 2: Representative photomicrographs from a sham animal showing the ROIs included for SμXRD (a,b,c) and histological (d,e,f) analyses at approximately -2.00 mm from bregma in the ipsilateral side of the brain.
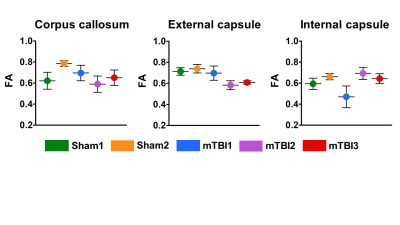
· Figure 3: Ex vivo FA metrics from the corpus callosum, external capsule and internal capsule in sham-operated and mTBI animals.
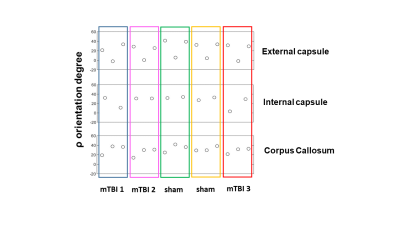
· Figure 4: Ex vivo XRD orientation degree from the Corpus callosum, external capsule and internal capsule in sham-operated and mTBI animals.