4498
Mapping transmit and receive B1 using variable flip angle acquisition on a person-by-person basis for hyperpolarized Carbon-13 and Xenon-129 MRI1Oxford Centre for Clinical Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 2Department of Radiology, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom, 3Department of Oncology, Univeristy of Oxford, Oxford, United Kingdom, 4RRPPS, University Hospitals Birmingham, Birmingham, United Kingdom, 5Oxford Respiratory Service, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom, 6Department of Physiology, Anatomy and Genetics, Univeristy of Oxford, Oxford, United Kingdom, 7GE Global Research, Munich, Germany, 8Department of Oncology, University of Oxford, Oxford, United Kingdom, 9Alama Mater Studorium, University of Bologna, Bologna, Italy
Synopsis
Keywords: Hyperpolarized MR (Gas), Hyperpolarized MR (Non-Gas), Xenon-129, Carbon-13
Hyperpolarized MRI allows the imaging of processes such as gas exchange and metabolism. Calibration of transmit and receive (Tx/Rx) B1 is required to account for coil inhomogeneities in reconstruction. A variable flip angle (VFA) method, which is fast and readily implemented, allows for a simultaneous B1/T1 measurement. In this study, a mathematical model of VFA calibration was developed, tested, validated in a hyperpolarized Carbon-13 (13C) phantom and in vivo with hyperpolarized Xenon-129 imaging of human lungs.Introduction
Hyperpolarization techniques can be employed to greatly increase the polarization of nuclei such as Xenon-129 [1] and Carbon-13[2], allowing for the imaging of processes such as gas exchange and metabolism[3]–[5].RF coil inhomogeneities and inter-person variability lead to the need for image correction to correctly reconstruct, and subsequently interpret, images. Amongst calibration schemes previously employed [6]–[8], the VFA method has the advantages of being fast, readily implemented, and does not require specialized software for scanner control [9]. The VFA method has previously been employed in pre-clinical experiments, however it has not been validated in human lung imaging, nor for hyperpolarized carbon-13 MRI [10]. The aim of this study was threefold: (1) to develop and test a mathematical model describing VFA calibration, (2) to validate T1 and B1 measurements in a 13C pyruvate phantom, with a well-validated ex vivo T1, and (3) to validate B1 mapping for Xenon imaging of the lung.
Methods
SimulationThe VFA calibration scheme was simulated using:
$$ S = M_0exp\left(-\frac{TR}{T_1}\right)^{TR\cdot Time_t}\cdot \sin(\Delta B_1\cdot \theta_{Time_t}) \prod_{Time_0}^{Time_t} \cos(\Delta B_1\cdot \theta_{Time_t}) $$
Where S is the detected signal, M0 is the magnetization, TR is the repetition time, θ is the flip angle at a given time during the experiment, Time is a vector of the time points during acquisition, T1 is the longitudinal relaxation time of the hyperpolarized species, and ΔB1 is the transmit B1 error which represents variation in B1 field.
M0, ΔB1, T1, TR and θ were varied and simulations corrupted with random noise. Each simulation was run 1000 times per perturbation in MATLAB (2022b, The Mathworks, Cambridge, MA).
Phantom
0.5g of [1-13C]pyruvate (Merck, USA) with 15mM AH111501 (Syncom, The Netherlands) was polarized for 3 hours in a SPINLab hyperpolarizer (GE Healthcare, WI)[11]. Data was acquired on a 3T Premier MRI scanner (GE Healthcare, WI) using a 1H/13C head coil (Rapid Biomedical, Germany). Transmit gain (TG) for 13C was calibrated using an ethylene-glycol sphere. The phantom was replaced with a custom 3D-printed falcon tube holder, with the tube filled with water for center frequency (f0) calibration [12]. Pyruvate was dissolved and ~8mL of NaOH buffer added, with water for injection used to fill the tube prior to insertion into the holder. Multi-flip angle pulse-acquire spectroscopy was acquired (Flip angle train (FAT) = 5x5 o, 5x10 o, 5x20 o, 5x30 o, TR = 1s, 1024 points, slice-selective pulse, and slice thickness = 20mm). Data was fit in MATLAB using non-linear least square fitting.
In vivo
4 participants (3 female, 1 male; mean age = 37 (+- SD=10.7)) were imaged. The study was approved by a local ethics committee and participants provided written informed consent. Enriched Xenon was polarized for ~10 minutes in a commercial polarizer (Polarean, USA). Participants inhaled 1L of hyperpolarized xenon and nitrogen (0.1:0.9L, respectively). Data was acquired with a Tx/Rx vest coil (PulseTeq, Cobham, UK) on a different 3T GE Premier system than the one used for 13C.
In the same breath hold, f0 and TG estimation was performed using a Bloch-Siegert acquisition [8] (1024 points, 32 scans, 75ms repetition time, hard pulse, bandwidth 20kHz), and then spiral imaging acquired (16-point spiral, with twelve 15 mm slices, FOV = 40 x 40mm, soft pulse, 32x32 reconstruction matrix, FAT = 6x10o, 6x20o, 6x30o, number of FAT = 2, TR = 230ms, total scan time = 8s). SNR masking (threshold = 10) was performed prior to fitting. Histograms of B1 and B1 corrected M0 images were generated.
Results
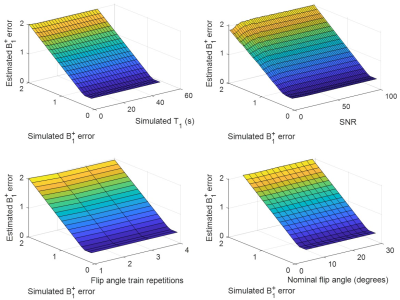
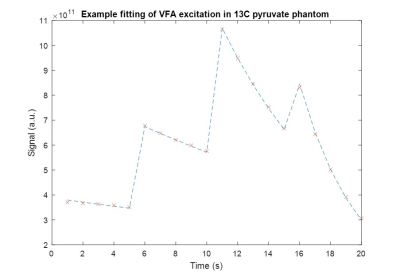
The model estimated B1 errors were in good agreement with the initial B1 errors used for simulation down to ΔB1 of 0.3. The estimated and simulated B1 errors had correlation coefficients of at least 0.995 (Fig. 1).Hyperpolarized 13C pyruvate T1 was found to be to be approximately 62s (Fit in Fig. 2), similar to literature values. The B1 error was found to be 0.7806.
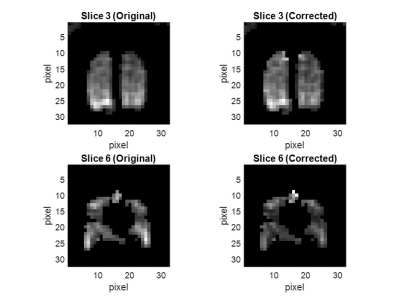
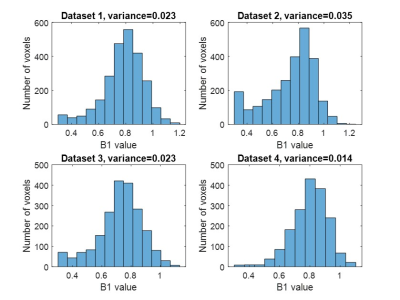
In vivo acquisitions were successful in all participants. B1 correction of M0 maps provided noticeable smoothing (Fig. 3). Fitting one slice took 1.18s. Histograms of B1, approximately Gaussian, are shown in Fig. 4.
Discussion
This study showed the robustness of the VFA method for mapping coil sensitivity on a person-by-person basis.Phantom tests show that the VFA scheme can be used to estimate B1 for Carbon-13 spectroscopy. This serves as a stepping-stone for B1 imaging, which may assist in modelling of metabolism[13].
The results from the 4 participants demonstrate that the VFA method can readily be used for clinical imaging. Other methods may use assumed coil maps, which may not fully reflect the profile of flexible coils[14], [15], so the VFA scheme is a promising alternative. With only a small amount of Xenon and a short time of 8 seconds, coil B1 can be successfully estimated, allowing integration into a Xenon workflow.
Conclusion
This study demonstrated that this VFA calibration scheme can be used for different hyperpolarized nuclei, achieving fast calibration of transmission gain. This will serve as an effective method for the optimization of hyperpolarization images, which can be easily integrated into the clinical workflow.Acknowledgements
This work was supported by the National Institute for Health and Care Research. Acknowledgement must also be given to Oxford-MRC Doctoral Training Partnership iCASE award and GE Healthcare for funding this project.References
[1] D. Raftery, E. MacNamara, G. Fisher, C. V. Rice, and J. Smith, “Optical Pumping and Magic Angle Spinning: Sensitivity and Resolution Enhancement for Surface NMR Obtained with Laser-Polarized Xenon,” J. Am. Chem. Soc., vol. 119, no. 37, pp. 8746–8747, Sep. 1997, doi: 10.1021/ja972035d.
[2] J. H. Ardenkjær-Larsen et al., “Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR,” Proc. Natl. Acad. Sci. U.S.A., vol. 100, no. 18, pp. 10158–10163, Sep. 2003, doi: 10.1073/pnas.1733835100.
[3] J. E. Roos, H. P. McAdams, S. S. Kaushik, and B. Driehuys, “Hyperpolarized Gas MRI: Technique and Applications,” Magn Reson Imaging Clin N Am, vol. 23, no. 2, pp. 217–229, May 2015, doi: 10.1016/j.mric.2015.01.003.
[4] M. Vaeggemose, R. F. Schulte, and C. Laustsen, “Comprehensive Literature Review of Hyperpolarized Carbon-13 MRI: The Road to Clinical Application,” Metabolites, vol. 11, no. 4, p. 219, Apr. 2021, doi: 10.3390/metabo11040219.
[5] P. E. Z. Larson and J. W. Gordon, “Hyperpolarized Metabolic MRI—Acquisition, Reconstruction, and Analysis Methods,” Metabolites, vol. 11, no. 6, Art. no. 6, Jun. 2021, doi: 10.3390/metabo11060386.
[6] K. Qing et al., “Regional mapping of gas uptake by blood and tissue in the human lung using hyperpolarized xenon-129 MRI: Lung MRI of Xe129 Uptake by Blood and Tissue,” J. Magn. Reson. Imaging, vol. 39, no. 2, pp. 346–359, Feb. 2014, doi: 10.1002/jmri.24181.
[7] M. H. Deppe, K. Teh, J. Parra-Robles, K. J. Lee, and J. M. Wild, “Slice profile effects in 2D slice-selective MRI of hyperpolarized nuclei,” Journal of Magnetic Resonance, vol. 202, no. 2, pp. 180–189, Feb. 2010, doi: 10.1016/j.jmr.2009.11.003.
[8] R. F. Schulte et al., “Transmit gain calibration for nonproton MR using the Bloch-Siegert shift,” NMR Biomed, vol. 24, no. 9, pp. 1068–1072, Nov. 2011, doi: 10.1002/nbm.1657.
[9] J. Zhong, W. Ruan, Y. Han, X. Sun, C. Ye, and X. Zhou, “Fast Determination of Flip Angle and T1 in Hyperpolarized Gas MRI During a Single Breath-Hold,” Sci Rep, vol. 6, no. 1, p. 25854, Sep. 2016, doi: 10.1038/srep25854.
[10] R. Healicon et al., “Assessing the effect of anesthetic gas mixtures on hyperpolarized 13 C pyruvate metabolism in the rat brain,” Magnetic Resonance in Med, vol. 88, no. 3, pp. 1324–1332, Sep. 2022, doi: 10.1002/mrm.29274.
[11] J. T. Grist et al., “Quantifying normal human brain metabolism using hyperpolarized [1–13C]pyruvate and magnetic resonance imaging,” NeuroImage, vol. 189, pp. 171–179, Apr. 2019, doi: 10.1016/j.neuroimage.2019.01.027.
[12] J. T. Grist et al., “Creating a clinical platform for carbon-13 studies using the sodium-23 and proton resonances,” Magnetic Resonance in Medicine, vol. 84, no. 4, pp. 1817–1827, 2020, doi: 10.1002/mrm.28238.
[13] C. J. Harlan, Z. Xu, C. M. Walker, K. A. Michel, G. D. Reed, and J. A. Bankson, “The effect of transmit B1 inhomogeneity on hyperpolarized [1-13C]-pyruvate metabolic MR imaging biomarkers,” Medical Physics, vol. 48, no. 9, pp. 4900–4908, 2021, doi: 10.1002/mp.15107.
[14] B. Driehuys et al., “Chronic Obstructive Pulmonary Disease: Safety and Tolerability of Hyperpolarized 129Xe MR Imaging in Healthy Volunteers and Patients,” Radiology, vol. 262, no. 1, pp. 279–289, Jan. 2012, doi: 10.1148/radiol.11102172.
[15] B. Driehuys, H. E. Möller, Z. I. Cleveland, J. Pollaro, and L. W. Hedlund, “Pulmonary Perfusion and Xenon Gas Exchange in Rats: MR Imaging with Intravenous Injection of Hyperpolarized 129Xe1,” Radiology, vol. 252, no. 2, pp. 386–393, Aug. 2009, doi: 10.1148/radiol.2522081550.
Figures