4489
Exploring the oscillating gradient waveform frequency dependence of diffusivity changes in human acute ischemic stroke1Biomedical Engineering, University of Alberta, Edmonton, AB, Canada, 2Neurology, University of Alberta, Edmonton, AB, Canada, 3Radiology and Diagnostic Imaging, University of Alberta, Edmonton, AB, Canada, 4Siemens Healthcare GmbH, Erlangen, Germany
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Microstructure
Two previous oscillating-gradient-spin-echo (OGSE) studies of human acute ischemic stroke using different diffusion waveforms (40 Hz, one-period; 50 Hz, two-period) have reported considerably different mean diffusivity (MD) changes in lesion white matter relative to long diffusion time (“0 Hz”) pulsed-gradient-spin-echo (PGSE). Here both OGSE waveforms were acquired in 8 stroke patients. Lesions showed marked diffusion time dependencies with lesion MD reduction of 40% for PGSE compared to 25% for OGSE 40Hz, and yet OGSE 50Hz was only a bit less (21%). Large heterogeneity between patients (+6% to -43% of OGSE 50Hz MD lesion changes) may explain earlier study differences.
Introduction
The use of oscillating-gradient-spin-echo (OGSE) diffusion MRI with short diffusion times (e.g. <5 ms), relative to pulsed-gradient-spin-echo (PGSE) with long diffusion times (e.g. ~40 ms), has shown less mean diffusivity (MD) reduction in the ischemic brain of animal models1-3 and human acute stroke4, 5. The two human stroke studies above reported disparate lesion white matter MD reductions. The first reported only 8% MD decrease over 11 patients using a two-period trapezoid cosine OGSE waveform (50 Hz, diffusion time ~4.3 ms)4, while the second reported a much more substantial 26% MD drop over 28 patients using a one-period trapezoid cosine OGSE waveform (40 Hz, diffusion time ~5.1 ms)5. Both studies report similar 37% and 35% MD reductions with PGSE. Given animal model observations that ischemic MD reduction depends on OGSE frequency1-3, the purpose here was to compare these two OGSE waveforms in the same stroke patients.Methods
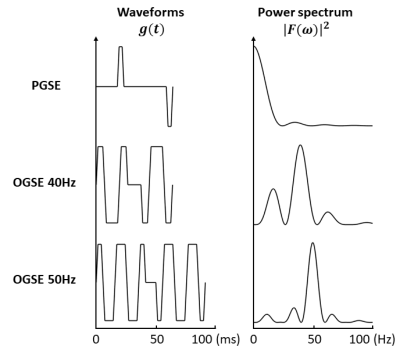
The 8 (sub)acute ischemic stroke patients were 66±6 (54-74) years old, 7 males, NIH stroke scale score 8±5 (1-18), scanned 42±22 (6-71.5) hours after stroke onset, and had lesion volumes of 5±7 (0.02-18.1) cm3. Six lesions had major white matter involvement in the posterior limb of the internal capsule (PIC) or corona radiata (CR) and two with only subcortical WM involvement. Images were acquired at 3T (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with 20 3mm (no gap) axial slices centred on the lesion, 1.85x1.85 mm2 in-plane resolution, GRAPPA R=2 with other core parameters shown in Table 1. Three diffusion waveforms similar to the two earlier studies4, 5 were acquired in 8 minutes total using a research sequence: (i) PGSE (b = 500 s/mm2), (ii) OGSE 40 Hz trapezoid cosine with 1 period per TE/2 and b = 500 s/mm2, and (iii) OGSE 50 Hz trapezoid cosine with 2 periods per TE/2 and b = 400 s/mm2 (Figure 1). All used 6 directions and 3 averages and b values were chosen to keep TE reasonable at 80 or 108 ms. Diffusion tensor imaging maps of mean, axial (AD), and radial diffusivity (RD) were computed using ExploreDTI and FSL. Manual regions-of-interest in white matter regions of the acute lesion and contralateral hemisphere were used for comparison of diffusion metrics between PGSE, OGSE 40Hz, and OGSE 50Hz, over the group of 8 patients as well as for examination of individual variability.Results
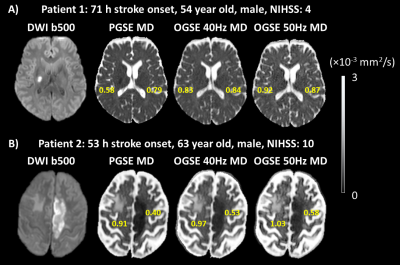
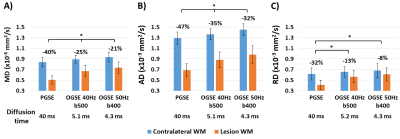
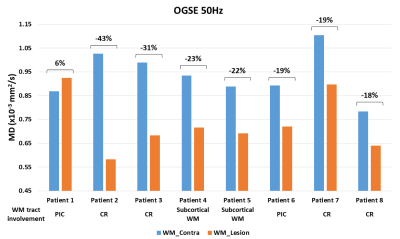
Lesion MD is less reduced for shorter diffusion times, but the degree of reduction varied between patients. For example, patient 1 with a lesion in the right PIC showed a distinct 27% MD reduction on PGSE which was not visibly different from contralateral tissue on the two OGSE scans (Figure 2A). In contrast, patient 2 with a lesion in left CR had a 56% MD reduction on PGSE with smaller, but still large MD reductions on OGSE 40Hz (45%) and OGSE 50Hz (43%) (Figure 2B). Over all 8 patients, lesion white matter showed progressive diffusion time effects with MD reductions (relative to contralateral white matter) of 40±9% for PGSE, 25±13% for OGSE 40Hz and 21±14% for OGSE 50Hz, with significant but small difference between one-period 40 Hz and two-period 50 Hz OGSE (Figure 3A). Notably, AD is the primary driver of reduced MD, dropping 47% relative to 32% for RD on PGSE, and 32% relative to 8% for RD on OGSE 50Hz. Lesion RD approaches the contralateral ‘healthy’ value on OGSE 50Hz. There is a large variability in lesion diffusivity change across the 8 patients, with 27-56% lower lesion MD for PGSE (relative to contralateral), and 6% greater to 43% lower lesion MD for OGSE 50Hz (Figure 4).Discussion
The 25% lower MD in stroke lesion white matter (compared to contralateral tissue) for 40 Hz OGSE (1 period) is consistent with our previous study (26% MD reduction) using the exact same protocol5. However, while the OGSE MD reduction at 50 Hz (2 periods) measured here (-21%) is significantly smaller than OGSE 40Hz, it is still considerably greater than the 8% reduction reported in reference [4]. Thus, the relative MD differences between two previous OGSE human stroke studies4, 5 cannot be explained by differences in diffusion spectrum, granted the frequencies were not very different (as opposed to the much higher frequencies attainable in animal models1-3). Although there may be other methodological differences (e.g. 4.7T vs 3T, different single-shot EPI implementation, b value = 300 s/mm2 in reference [4]), the large heterogeneity in relative MD difference between lesion and contralateral tissue demonstrated here in Figures 2 and 4 suggests that the variability may lie in patient or lesion location differences. Greater diffusion time effects are known to be present in white matter regions with larger (healthy) axon diameters6, and thus study average results could be influenced by the proportion of stroke patients with large axon diameter lesions. Finally, it should be noted that although the absolute values of lesion diffusivity change for OGSE 50Hz measured here are greater than in reference [4], the trend of much smaller RD (8%) than AD (32%) change yields good agreement with reference [4] and the explanation of axonal beading driving lesion diffusivity change in human acute ischemic stroke4, 7.Acknowledgements
Supported by the Heart and Stroke Foundation of Canada and China Scholarship Council.References
1. Aggarwal M, Burnsed J, Martin LJ, Northington FJ, Zhang J. Imaging neurodegeneration in the mouse hippocampus after neonatal hypoxia–ischemia using oscillating gradient diffusion MRI. Magnetic resonance in medicine. 2014;72(3):829-40.
2. Does MD, Parsons EC, Gore JC. Oscillating gradient measurements of water diffusion in normal and globally ischemic rat brain. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine. 2003;49(2):206-15.
3. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating gradient diffusion MRI reveals unique microstructural information in normal and hypoxia‐ischemia injured mouse brains. Magnetic resonance in medicine. 2014;72(5):1366-74.
4. Baron CA, Kate M, Gioia L, Butcher K, Emery D, Budde M, Beaulieu C. Reduction of diffusion-weighted imaging contrast of acute ischemic stroke at short diffusion times. Stroke. 2015;46(8):2136-41.
5. Zhou M, Stobbe R, Buck B, Butcher K, Fairall P, Boyd A, Emery D, Feiweier T, Beaulieu C. Heterogeneous diffusivity changes within human stroke lesions measured with oscillating gradient spin echo diffusion MRI. Proc Int Soc Magn Reson Med London UK. 2022;0143.
6. Xu J, Li H, Harkins KD, Jiang X, Xie J, Kang H, Does MD, Gore JC. Mapping mean axon diameter and axonal volume fraction by MRI using temporal diffusion spectroscopy. Neuroimage. 2014;103:10-9.
7. Budde MD, Frank JA. Neurite beading is sufficient to decrease the apparent diffusion coefficient after ischemic stroke. Proceedings of the National Academy of Sciences. 2010;107(32):14472-7.
Figures
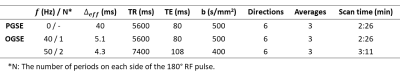
Table 1: Protocol parameters for PGSE with 40 ms effective diffusion time (0 Hz) and shorter diffusion time OGSE 40Hz (diffusion time of 5.1 ms) and OGSE 50Hz (diffusion time of 4.3 ms).