4488
Modelling transverse relaxometry using myelin-induced off-resonance fields and magnetisation transfer
1Wellcome Center for Integrative Neuroimaging (WIN), University of Oxford, Oxford, United Kingdom
Synopsis
Keywords: Microstructure, Microstructure
We present a new Monte Carlo MR simulator, which in addition to simulating the random diffusion of water restricted by any obstacles also simulates (1) the off-resonance field due to myelin and (2) signal loss due to magnetisation transfer. We use this simulator to study the interaction of diffusion, relaxation, and magnetisation transfer. We simulate gradient and spin echo measurements in white matter. The myelin off-resonance field is found to greatly reduce T2* due to dephasing of the signal between compartments, but barely affects T2. Magnetisation transfer at tissue boundaries explains T2 differences between intra-, extra-axonal, and myelin water.
Introduction
MRI signal evolution is affected by interactions between spins and the magnetic field imposed by both the MR scanner sequence and the magnetic susceptibility of the surrounding tissue. Modelling how these contribute to MRI signal evolution is complicated by the random diffusion of spins, which causes each spin to probe different parts of the tissue microstructure.While the effect of diffusion is integral to models of the diffusion MRI signal, it is typically ignored in the modelling of other MRI modalities, such as relaxometry. In these modalities the water is usually considered well mixed either throughout the tissue (e.g., the water pool in magnetisation transfer1) or well mixed within individual tissue compartments (e.g., intra-axonal, extra-axonal, and myelin water2). Conversely, diffusion MRI models typically ignore the effects of T2 relaxation or magnetisation transfer, even though they can affect the diffusion MRI attenuation3.
Here we combine diffusion and the effects of the local microstructure on the magnetisation in a single simulator. We use this to ask whether magnetisation transfer and the myelin-induced off-resonance field can explain the much shorter T2 and T2* estimated in brain tissue compared with cerebrospinal fluid (CSF).
Methods
We developed a new Monte Carlo MR simulator written in Julia4 (MCMRSimulator.jl v0.3). The simulator follows the random walk of thousands of spins. These spins can collide with user-defined tissue microstructure formed of cylinders (axons), spheres (somas), or arbitrary meshes (any microstructure)5. During their random walk the spin magnetisation is tracked, which will be influenced by the sequence (user-defined from arbitrary RF pulses and gradient waveforms) and the local tissue.In this abstract we simulate white matter tissue as a random configuration of cylinders (axons) surrounded by myelin sheaths (Fig.1). Impermeable cylinders separate the myelin space from the intra- and extra-axonal spaces preventing any exchange between these compartments.
The transverse signal evolution in these simulations is affected by (Fig.1):
- T2 relaxation time of 2 seconds matching CSF6. Within the tissue this T2 will be shortened by magnetisation transfer and myelin-induced off-resonance.
- 0.5% of the magnetisation is transferred at each membrane collision (Figure 1). Due to short T2 of the bound water pool7 this transfer corresponds to a transverse signal loss of 0.5% per collision. This transfer rate was chosen to match the expected tissue T2.
- Dephasing due to the myelin-induced off-resonance field. For these infinitely long axons, the myelin-induced off-resonance field is given by the hollow fibre model8, which models both isotropic ($$$\chi_I$$$) and anisotropic ($$$\chi_A$$$) components of the myelin magnetic susceptibility.
Simulation results
Spin echo and T2:In the spin echo sequences, the myelin-induced off-resonance field was only a minor contributor to the decrease in T2 in tissue compared with CSF, with the main decrease being caused by magnetisation transfer in collisions with the membranes (Fig.2A).
While the match between simulated and observed tissue T2 is unsurprising given that the magnetisation transfer rate was set to match the T2, it is more interesting that we can reproduce the correct trend in T2’s across the tissue compartments (Table 1). While the myelin T2 is still overestimated, this might improve by more accurately modelling myelin wraps.
The transverse relaxation of the signal summed across multiple compartments (solid lines in Fig.2) is simply a weighted sum of the relaxation within individual compartments (dashed lines in Fig.2).
Gradient echo and T2*:
In addition to the irreversible T2 relaxation due to magnetisation transfer discussed above, the gradient echo signal has signal loss due to dephasing caused by the myelin-induced off-resonance field (Fig.3A). This dephasing is dominated by the field differences between compartments rather than the field differences within compartment as can be seen by the long T2* even in myelin water (Fig.3). Due to a chance realignment between compartments, the signal partially recovers at long echo times (Fig.3B).
Varying microstructure
In this model the T2* is mainly dependent on myelin-induced off-resonance field and hence would increase in cases of demyelination (Fig.4A) or a smaller angle between the axons and the main magnetic field (Fig.4B) in line with previous models and observations12. The T2 is mainly affected by the collision rate, which is reduced extra-axonally due to demyelination (Fig.4A), larger axons (Fig.4C) or lower axon density (Fig.4D). Meanwhile the intra-axonal T2 increases for larger axons (Fig.4C), which might explain the large intra-axonal T2 measured in the corticospinal tract13. An orientation-dependent magnetisation transfer rate would need to be modelled to get an orientation-dependent T214 (Fig.4B).
Discussion
Combining diffusion and other MR physics in a single Monte Carlo simulator allows these simulations to be used for more than modelling diffusion MRI. While such a simulator cannot fully account for effects on a nanoscale (e.g., spin-spin interactions) or macroscale (e.g., head shape), we show that by simply including myelin-induced off-resonance fields and magnetisation transfer effects, we can reproduce many features of transverse relaxation.The myelin-induced off-resonance field explains the short T2* in myelinated tissue (Fig.2) including its dependence on fibre orientation (Fig.4)12. Magnetisation transfer can explain the T2 differences between intra-axonal, extra-axonal, and myelin water (Table 1). We plan to further develop the simulator to include more realistic tissue-spin interactions and match more quantitative MRI modalities.
MCMRSimulator.jl documentation: https://open.win.ox.ac.uk/pages/ndcn0236/mcmrsimulator.jl/dev/
Reproducible notebook: https://users.fmrib.ox.ac.uk/~ndcn0236/notebooks/gradient_spin_echo.html
Acknowledgements
We thank Amy Howard, Ben Tendler, and Karla Miller for helpful discussions and comments on this abstract. Michiel Cottaar and Saad Jbabdi are funded by a Wellcome Collaborative Award (grant no. 215573/Z/19/Z) and a Wellcome Senior Research Fellowship (grant no. 221933/Z/20/Z). The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (grant no. 203139/Z/16/Z).References
1. Henkelman, R. M. et al. Quantitative interpretation of magnetization transfer. Magn Reson Med 29, 759–66 (1993).
2. Drakesmith, M., Kleban, E., Fabrizio, F. & Jones, D. K. Improved estimates of the g-ratio by modelling its contribution to complex signal evolution in GRE data. ISMRM, 4897 (2019).
3. Lampinen, B. et al. Searching for the neurite density with diffusion MRI: Challenges for biophysical modeling. Hum Brain Mapp 40, 2529–2545 (2019).
4. Bezanson, J., Edelman, A., Karpinski, S. & Shah, V. B. Julia: A fresh approach to numerical computing. SIAM review 59, 65–98 (2017).
5. Hall, M. G. & Alexander, D. C. Convergence and parameter choice for Monte-Carlo simulations of diffusion MRI. IEEE Trans Med Imaging 28, 1354–64 (2009).
6. Spijkerman, J. M., Petersen, E. T., Hendrikse, J., Luijten, P. & Zwanenburg, J. J. M. T2 mapping of cerebrospinal fluid: 3 T versus 7 T. MAGMA 31, 415–424 (2018).
7. Morrison, C. & Henkelman, R. M. A model for magnetization transfer in tissues. Magnetic Resonance in Medicine 33, 475–482 (1995).
8. Wharton, S. & Bowtell, R. Fiber orientation-dependent white matter contrast in gradient echo MRI. Proc. Natl. Acad. Sci. U.S.A. 109, 18559–64 (2012).
9. McKinnon, E. T. & Jensen, J. H. Measuring intra‐axonal T2 in white matter with directionaveraged diffusion MRI. Magn Reson Med 81, 2985–2994 (2019).
10. Veraart, J., Novikov, D. S. & Fieremans, E. TE dependent Diffusion Imaging (TEdDI) distinguishes between compartmental T2 relaxation times. NeuroImage 182, 360-369 (2018).
11. Lee, J. et al. So You Want to Image Myelin Using MRI: An Overview and Practical Guide for Myelin Water Imaging. Journal of Magnetic Resonance Imaging 53, 360–373 (2021).
12. Kor, D. et al. The role of iron and myelin in orientation dependent R2* of white matter. NMR in Biomedicine 32, e4092 (2019).
13. Lampinen, B. et al. Towards unconstrained compartment modeling in white matter using diffusion-relaxation MRI with tensor-valued diffusion encoding. Magn Reson Med 84, 1605-1623, (2020).
14. Pampel, A., Müller, D. K., Anwander, A., Marschner, H. & Möller, H. E. Orientation dependence of magnetization transfer parameters in human white matter. Neuroimage 114, 136–46 (2015).
Figures
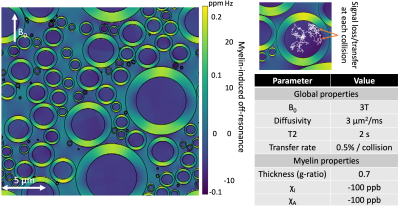
Figure 1 Random configuration of infinitely repeating cylinders representing axons (left). Each axon is surrounded by a myelin sheath with the colour giving off-resonance field generated by the myelin’s magnetic susceptibility. Spin particles follow a random walk obstructed by the myelin sheath (upper right). The simulated spin evolution is affected by the MR sequence, the local myelin-induced off-resonance field, and a transverse signal loss at each collision due to magnetisation transfer. Simulation parameters are summarised in the lower right.
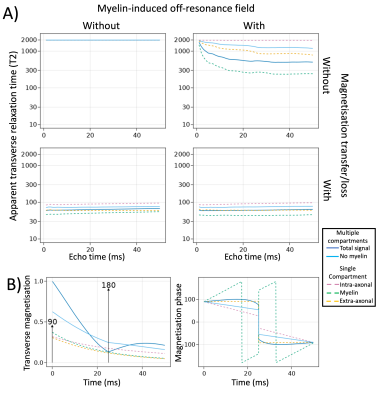
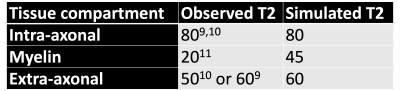
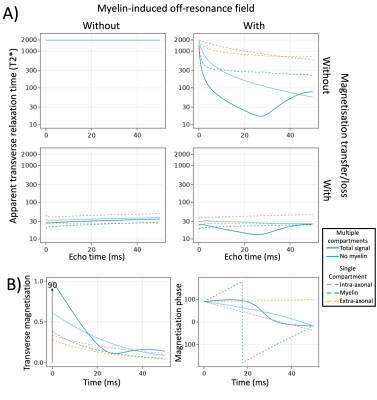
Figure 3 A) T2* estimates (y-axis) at various echo times (x-axis) with or without myelin-induced off-resonance fields (columns) and with or without magnetisation transfer (rows). B) Signal evolution in a single gradient echo sequence (TE=50ms) showing the magnitude (left) or phase (right) of the transverse magnetisation. Different lines show the signal is summed across compartments (solid) or within each compartment (dashed). The T2* relaxation is driven by myelin-induced dephasing between compartments.
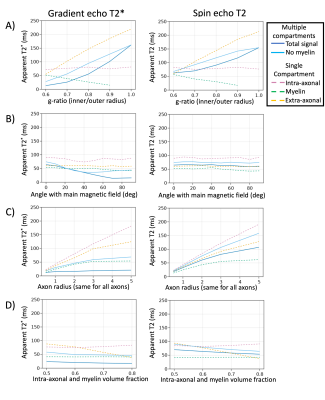
Figure 4 The T2* of a gradient echo sequence (left) and the T2 of a spin echo sequence plotted as a function of various microstructural parameters. Both sequences have an echo time of 30 ms: A) Demyelination where the myelin sheath is replaced by extra-axonal water (less myelin to the right); B) Angle with the main magnetic field (orthogonal on the right); C) axon radius; D) fibre density.