4482
Diffusion filters, T2-filters, diffusion tensor component diffusivities, and compartmental diffusivities. What is happening in FEXI?1Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Research Institute, Baltimore, MD, United States, 3Department of Medical Radiation Physics, Lund University, Lund, Sweden, 4Deparment of Radiology, Clinical Sciences Lund, Lund University, Lund, Sweden
Synopsis
Keywords: Diffusion/other diffusion imaging techniques, Diffusion/other diffusion imaging techniques, FEXI, trans-membrane exchange
We explored how compartmental anisotropy affects filter-exchange imaging (FEXI) data in white matter by applying different types of tissue water magnetization filters, including multi-orientation and spherical tensor encoded diffusion filters, as well as a T2 filter. The results show that, depending on the mutual orientation of the filter and detection gradient pairs relative to fiber orientation, filtering efficiencies can be positive or negative. We conclude that the fast compartment reduced during diffusional filtering need not be extracellular, but can also reflect intra-axonal signal losses with effect size determined by the diffusion tensor components perpendicular and parallel to the fibers.Introduction
$$${\;\;\;\;}$$$Trans-membrane exchange can be an important indicator of tissue functioning. To quantify cellular trans-membrane exchange using MRI, Lasic et al.1 proposed the filter-exchange imaging (FEXI) technique for measuring exchange between slow and fast diffusion compartments. Based on in-vitro cell experiments2,3, the FEXI method assumes that diffusion is slower inside the cell than outside and that the diffusion filter suppresses the extracellular compartment. Apparent exchange rates (AXRs) measured by FEXI have been reported to distinguish abnormalities in tumor tissue4 and subtypes5,6.$$${\;\;\;\;}$$$Recently, it was demonstrated that AXR is affected not only by filter strength7 but also diffusion anisotropy, especially in white matter8,9. This indicates that diffusion tensor components may be relevant in addition to diffusion compartments. Here, we explore how compartmental anisotropy affects FEXI results in white matter by applying different filtering and detection gradient orientations. We also investigate the effect of T2 filtering alone, i.e., with zero diffusion filter strength.
Methods
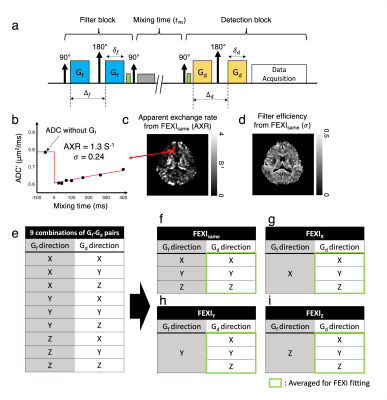
$$${\;\;\;\;}$$$The FEXI signal from a double-pulsed-gradient-spin-echo (PGSE) sequence (Fig. 1a) is1,4:$${S(b_d,t_m)\;=\;S'(t_m)exp(-b_d{\cdot}ADC'(t_m))\;\;\;\;\;\;[Eq.1]\\ADC'(t_m)\;=\;ADC\cdot(1-{\sigma}exp(-t_m{\cdot}AXR)\;\;\;\;\;\;[Eq.2]\\{\sigma}\;=\;(D_{fast}-D_{slow})(f_{fast}^{eq}-f_{fast}^0)/ADC\;\;\;\;\;\;[Eq.3]\\}$$ $$${ADC',\;t_m,\;{\sigma},\;and\;f_{fast}^{eq/0}\;}$$$indicate ADC measured during detection (Fig. 1b), mixing time, filtering efficiency, fast-/slow-diffusion coefficients, and equilibrium/filtered fractions of the fast-diffusing compartment, respectively. To estimate ADC’(tm), diffusion-weighted signals were voxel-wise fitted to Eq. 1; and AXR were estimated using Eq.2 (Fig.1).$$${\;\;\;\;}$$$Human studies were approved by the local IRB; all volunteers signed informed consent. Data were acquired at 3T (Philips Ingenia-Elition-RX, 95mT/m) with TE=45ms, 5mm single-slice, resolution=3×3mm2, relaxation-delay=4000ms, 4 averages, σf/σd/Δf/Δd=13.2/9.8/16.2/21.9ms, b-value for diffusion filter (bf)=900s/mm2, b-value for detection (bd)={10,300,600,900}s/mm2; ={15,100,200,400}ms. Detection gradient (Gd) was applied along X,Y, and Z for each filter gradient direction (Gf =X,Y,Z), giving 9 combinations (Fig.1e). Other experiments included spherical-b-tensor encoding for trace-weighted diffusion filtering (bf=900s/mm2)10,11 (Fig.4c), and omitting Gf for T2 filtering only (Fig.4e), both acquired with separate orthogonal diffusion readouts (Gd=X,Y,Z) to generate trace-weighted images.
$$${\;\;}$$$Images from different acquisitions were co-registered linearly. Trace images from the same Gf and Gd directions4 (FEXIsame;Fig.1f) and individual X, Y, Z Gf directions (FEXIX/Y/Z; Fig.1g-i) were generated by averaging DWIs acquired from three Gd directions and used for FEXI fitting.
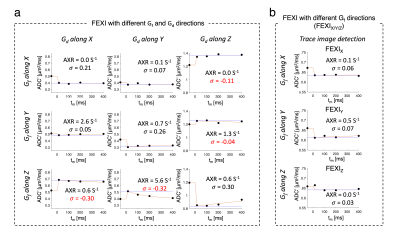
$$${\;\;\;\;}$$$ROI analysis was performed in cortical spinal tract, CST, for coherently oriented white matter fibers along Z-direction (defined as having an angle <15° between the principal eigenvector and Z)12.
Results
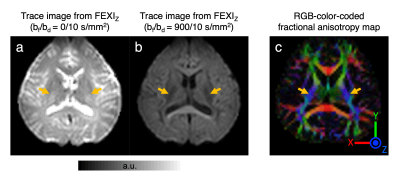
$$${\;\;\;\;}$$$When reconstructing FEXI using FEXIsame, frontal white matter results (AXR = 1.3 s-1 and σ = 0.24; Fig.1) are comparable to the literature.$$${\;\;\;\;}$$$Fig.2 shows FEXI parameters for CST where fiber is parallel to Z direction. Importantly, individual combinations of Gf and Gd directions show strong effects of tissue diffusional anisotropy. When applying Gf and Gd with the same direction relative to the fiber orientation (i.e. both perpendicular or both parallel), σ values are positive as expected [Eq. 3], while five of six AXR values are small (0-0.7s-1, Fig.2a), agreeing with a previous report9. Interestingly, when the direction relative to the fiber differs between Gf and Gd, negative σ values are measured consistently (Fig. 2a). Since, according to the FEXI model, σ reflects the relative saturation of the fast and slow diffusion components, this important observation would indicate that the slow diffusion component is filtered out more strongly than the fast component. This seemingly “impossible” effect is direct experimental evidence that, at least for FEXI in white matter, the description needs to be adjusted to account for the signal loss due to selective filtering of diffusion tensor components of at least two restricted compartments with a different diffusion tensor. This interpretation is supported by small σ-values when using trace-weighted detection (σ < 0.07) (Fig.2b). Also, when studying DWIs, filtering along the fiber direction shows strong signal reduction, indicating more filtering of intra-axonal signal than extra-cellular (Fig.3).
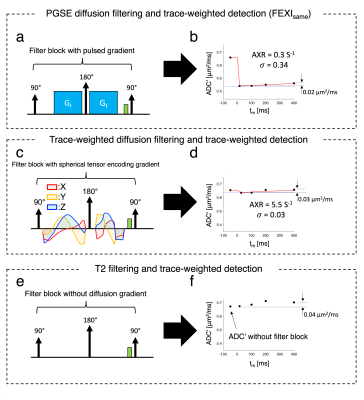
$$${\;\;\;\;}$$$Fig.4 compares three filter types, all with trace-weighted (X,Y,Z-averaged) detection: (a,b) PGSE-trace-weighted diffusion-filter (FEXIsame); (c,d) spherically-encoded diffusion-filter; (e,f) T2-filter. Regardless of filter type, the results show a comparable ADC' dependence on tm (ΔADC'=0.02-0.04μm2/ms) despite the strongly varying filter efficiencies σ (Fig.4). For the single-shot spherical-tensor-encoded trace-filter, σ is small compared to the PGSE-trace-filter based on averaging over separately applied gradient directions (X,Y,Z) (Figs.4b vs. d), in line with predictions by Khateri et al.13 These data suggest that to obtain measurable FEXI exchange in white matter, it is necessary to apply anisotropic diffusion filters to label the two compartments separately.
$$${\;\;\;\;}$$$The T2-filtering efficiency was negligible, i.e. ADC’ values with and without filter were equal, but ADC' still increased as a function of tm (Fig.4f). Such an increase may indicate removal of slower diffusing components within a compartment, possibly due to faster T1 decay during tm. This ADC’ evolution observed after applying a T2 filter of length equal to the echo time of the diffusion filter is likely to strongly affect AXR estimations.
Conclusion and discussion
$$${\;\;\;\;}$$$We explored the effects of diffusion anisotropy and T2-filtering on FEXI parameters in white matter. The results indicate that FEXI experiments in white matter need to account for and exploit differences in anisotropy between the exchanging diffusion compartments. FEXI measurements in the diseased brain need to be interpreted carefully because the physical and cellular changes in a pathological condition can alter the contribution diffusion anisotropy in tissue.Acknowledgements
No acknowledgement found.References
1. Lasič S, Nilsson M, Lätt J, Ståhlberg F, Topgaard D. Apparent exchange rate mapping with diffusion MRI. Magnet Reson Med 2011; 66:356–365.
2. Andrasko J. Water diffusion permeability of human erythrocytes studied by a pulsed gradient NMR technique. Biochimica Et Biophysica Acta Bba - Gen Subj 1976; 428:304–311.
3. Zijl PCV, Moonen CT, Faustino P, Pekar J, Kaplan O, Cohen JS. Complete separation of intracellular and extracellular information in NMR spectra of perfused cells by diffusion-weighted spectroscopy. Proc National Acad Sci 1991; 88:3228–3232.
4. Nilsson M, Lätt J, Westen D van, et al. Noninvasive mapping of water diffusional exchange in the human brain using filter‐exchange imaging. Magnet Reson Med 2013; 69:1572–1580.
5. Lampinen B, Szczepankiewicz F, Westen D van, et al. Optimal experimental design for filter exchange imaging: Apparent exchange rate measurements in the healthy brain and in intracranial tumors. Magnet Reson Med 2017; 77:1104–1114.
6. Lasič S, Oredsson S, Partridge SC, et al. Apparent exchange rate for breast cancer characterization. Nmr Biomed 2016; 29:631–639.
7. Bai R, Li Z, Sun C, Hsu Y-C, Liang H, Basser P. Feasibility of filter-exchange imaging (FEXI) in measuring different exchange processes in human brain. Neuroimage 2020; 219:117039.
8. Sønderby CK, Lundell HM, Søgaard LV, Dyrby TB. Apparent exchange rate imaging in anisotropic systems. Magnet Reson Med 2014; 72:756–762.
9. Li Z, Pang Z, Cheng J, et al. The direction-dependence of apparent water exchange rate in human white matter. Neuroimage 2022; 247:118831.
10. Szczepankiewicz F, Westin C-F, Nilsson M. Gradient waveform design for tensor-valued encoding in diffusion MRI. J Neurosci Meth 2021; 348:109007.
11. Sjölund J, Szczepankiewicz F, Nilsson M, Topgaard D, Westin C-F, Knutsson H. Constrained optimization of gradient waveforms for generalized diffusion encoding. J Magn Reson 2015; 261:157–168.
12. Fieremans E, Jensen JH, Helpern JA. White matter characterization with diffusional kurtosis imaging. Neuroimage 2011; 58:177–188.
13. Khateri M, Reisert M, Sierra A, Tohka J, Kiselev VG. What does FEXI measure? Nmr Biomed 2022:e4804.
Figures